A Profile of Pediatric Solid Tumors: A Single Institution Experience in Kashmir
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(04): 471-477
DOI: DOI: 10.4103/ijmpo.ijmpo_95_16
Abstract
Aims: The purpose of this retroprospective study was to study the epidemiological characteristics and outcomes of children with solid tumors at our institution. Subjects and Methods: Three hundred and three pediatrics patients registered at Regional Cancer Centre (RCC), Sher-i-Kashmir Institute of Medical Sciences (SKIMS), Srinagar, Kashmir, between January 2008 and June 2014, were analyzed with regard to demographic status, presentingcomplaints, investigations, treatment, morbidity, and outcomes. Standard statistical methods were used for analysis. Results: Among 19,880 patients registered at RCC, SKIMS from January 2008 till June 2014, 986 (4.9%) were of pediatric age group. Of these, 303 (30.7%) patients had pediatric solid tumors. The male-to-female ratio was 1.04, there were no infants (up to 27 days), 6% were infants and toddlers (28 days–23 months), 39% were children (2–11 years), and 55% were adolescents (12–19 years). There were 86% rural patients and 14% urban patients. Mostcommon were central nervous system tumors (25.74%), followed by germ cell tumors (14.52%), primitive neuroectodermal tumor/Ewing sarcoma (13.86%), Wilms' tumor (8.9%), osteosarcoma (6.6%), rhabdomyosarcoma (5.6%), colorectal cancer (5.28%), neuroblastoma (4.9%), and retinoblastoma (2.6%). Outcomes: 33.9% patients went into remission, 35.64% were defaulters, 2.97% had stable disease, 2.31% had partial response, 20.79% expired, and 3.96% were still on treatment. Of all these patients, 5.28% had a relapse. Conclusions: Across the series, advanced stage of presentation, a high incidence of default and poor follow-up was seen. Multiple interrelated factors are responsible for the poorer outlook of childhood cancer in Kashmir.
Publication History
Article published online:
04 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used forcommercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Aims:
The purpose of this retroprospective study was to study the epidemiological characteristics and outcomes of children with solid tumors at our institution.
Subjects and Methods:
Three hundred and three pediatrics patients registered at Regional Cancer Centre (RCC), Sher-i-Kashmir Institute of Medical Sciences (SKIMS), Srinagar, Kashmir, between January 2008 and June 2014, were analyzed with regard to demographic status, presenting complaints, investigations, treatment, morbidity, and outcomes. Standard statistical methods were used for analysis.
Results:
Among 19,880 patients registered at RCC, SKIMS from January 2008 till June 2014, 986 (4.9%) were of pediatric age group. Of these, 303 (30.7%) patients had pediatric solid tumors. The male-to-female ratio was 1.04, there were no infants (up to 27 days), 6%-were infants and toddlers (28 days–23 months), 39%-were children (2–11 years), and 55%-were adolescents (12–19 years). There were 86%-rural patients and 14%-urban patients. Most common were central nervous system tumors (25.74%), followed by germ cell tumors (14.52%), primitive neuroectodermal tumor/Ewing sarcoma (13.86%), Wilms' tumor (8.9%), osteosarcoma (6.6%), rhabdomyosarcoma (5.6%), colorectal cancer (5.28%), neuroblastoma (4.9%), and retinoblastoma (2.6%). Outcomes: 33.9%-patients went into remission, 35.64%-were defaulters, 2.97%-had stable disease, 2.31%-had partial response, 20.79%-expired, and 3.96%-were still on treatment. Of all these patients, 5.28%-had a relapse.
Conclusions:
Across the series, advanced stage of presentation, a high incidence of default and poor follow-up was seen. Multiple interrelated factors are responsible for the poorer outlook of childhood cancer in Kashmir.
Introduction
Cancer in children and adolescents is rare although the overall incidence of childhood cancer has been slowly increasing since 1975.[1] Worldwide, the annual number of new childhood cancer exceeds 200,000 and >80%-of these are from the developing world.[1] Seven out of 10 children with cancer in the resource rich countries are cured, with 5-year survival rates for certain cancers such as Hodgkin's lymphoma and retinoblastoma approaching 95%.[2,3] Recent studies have shown that this success in survival can be replicated in the developing world through shared expertise.[4,5,6,7] Childhood cancer remains the leading cause of disease-related mortality in children.[8] Malignant solid tumors account for approximately 30%-of childhood cancers.[9] The predominant histology of specific solid tumors varies significantly with age.[10]
Dramatic improvements in survival have been achieved in children and adolescents with cancer. Between 1975 and 2010, childhood cancer mortality decreased by >50%.[11] This success can be attributed to several factors. These include enrollment of patients into well-designed prospective clinical trials, systematic collection of tissue to better define the biology of disease, availability of more effective chemotherapy agents, use of multimodal therapy, better supportive care, and more refined diagnostic imaging methods that accurately define the extent of disease.
Data regarding cancer incidence are important for several reasons. Cancer affects all nations, and therefore, it is an endemic disease with considerable variation in frequency according to the site incidence. The geographical differences in total and site incidences have provided clues of causative factors and especially in separating environmental and ethnic factors from intrinsic factors. As we make progress in reducing infection-related childhood deaths in India, it is imperative to give attention to children with cancer, who have an increasing likelihood of cure with appropriate treatment.
Sher-i-Kashmir Institute of medical sciences (SKIMS), Srinagar is a 700-bedded tertiary care teaching hospital in Jammu and Kashmir. A Regional Cancer Centre (RCC) was established at SKIMS, under the national cancer control program with the objective to provide cancer treatment facilities in addition to cancer prevention programs across the state. In this study, we aimed to retroprospectively study the epidemiological characteristics, treatments received, and outcomes of children with solid tumors at RCC, SKIMS. Based on our study, we could better evaluate the status of our efforts to treat this subgroup of patients and deliver improved care.
Subjects and Methods
A retroprospective study was conducted in 303 pediatric patients (children and adolescents up to 19 years of age) with histologically proven solid tumors who were registered at RCC, SKIMS, Srinagar, between January 2008 to June 2014. A research protocol for this study was approved by the local Ethics Committee and informed consents were taken from each patient's parent/guardian.
A pro forma was developed, and patient characteristics with regard to age, sex, locality, residence, type of family, socioeconomic status, clinical presentation, investigations, treatment prescribed, and any morbidity in each case were studied in detail.
Following completion of treatment, the patients were followed up in our outpatient department. The frequency of follow-up visits was according to their individual tumor type and general recommendations. In each visit, a detailed history and physical examination and relevant investigations were done to detect any late side effects of treatment or relapse. The last follow-up studied was till January 2015.
Standard statistical methods were used for statistical analysis.
Results
Demographic data
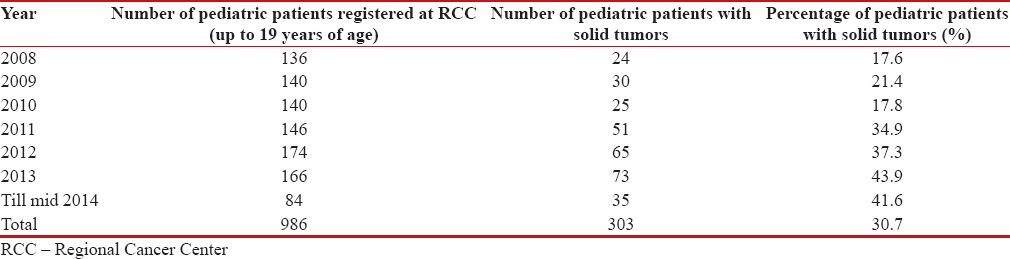
A total of 19,880 patients were registered at RCC, SKIMS, from January 2008 till June 2014. Among these patients, 986 (4.9%)-were of pediatric age group. And of these, 303 (30.7%) patients had pediatric solid tumors [Table 1].
Table 1
Pediatric patients registered at Regional Cancer Centre, Sher-i-Kashmir Institute of medical sciences from January 2008 to June 2014
|
All subsequent data are for the patients with pediatric solid tumors. There were 51%-male and 49%-females (M:F ratio was 1.04:1) [Figure 1]. There were no infants (up to 27 days), 6%-were infants and toddlers (28 days–23 months), 39%-were children (2–11 years), and 55%-were adolescents (12–19 years). There were 86%-rural patients and 14%-urban patients. Most patients (47%)-were from Budgam district followed by Baramulla (44%) and Srinagar district (43%).
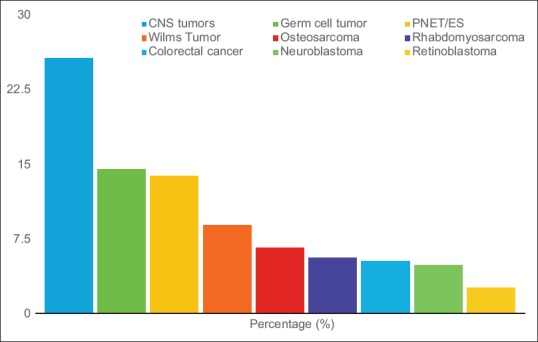
| Figure 1:Age distribution
Tumor distribution
The most frequent were central nervous system (CNS) tumors (25.74%) followed by (in descending order of frequency) germ cell tumors (14.52%), primitive neuroectodermal tumor (PNET)/Ewing sarcoma (ES) (13.86%), Wilms' tumor (8.9%), osteosarcoma (6.6%), rhabdomyosarcoma (5.6%), colorectal cancer (5.28%), neuroblastoma (4.9%), and retinoblastoma (2.6%) [Table 2 and Figure 2].
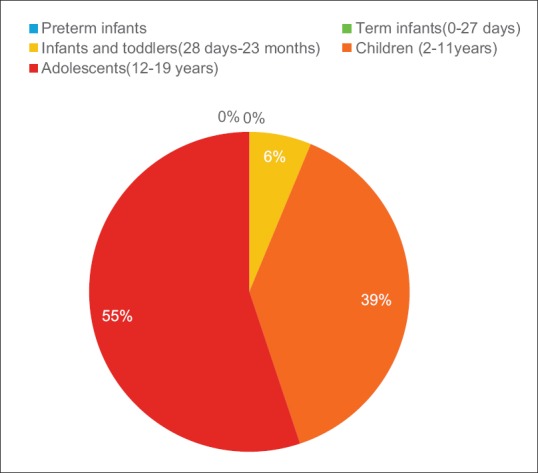
| Figure 2:Major tumor distribution
Table 2
Major tumor distribution

|
Outcome
Among the 303 patients studied, 33.9%-patients went into remission, 35.64%-were defaulters, 2.97%-had stable disease, 2.31%-had partial response, 20.79%-expired, and 3.96%-were still on treatment. Of all these patients 5.28%-had a relapse [Table 3].
Table 3
Overall outcomes

|
Morbidity
Among the documented morbidities, the most common was febrile neutropenia (9.2%) followed by chronic hepatitis B (1.6%).
Individual tumor characteristics
- Table 4 summarizes the characteristics of pediatric solid tumors in our study population CNS tumors (n-78): These were more frequent in males (59%) and in adolescents (55%). The most common histology was astrocytoma (25.64%) and WHO Grade IV (26.9%) though in 42.3%-cases grade was not mentioned. Most patients defaulted (48.7%), remission was documented in 16.6%, stable disease in 1.2%, partial response in 6.4%, and 23%-expired
Table 4
Characteristics of the pediatric solid tumors in the study population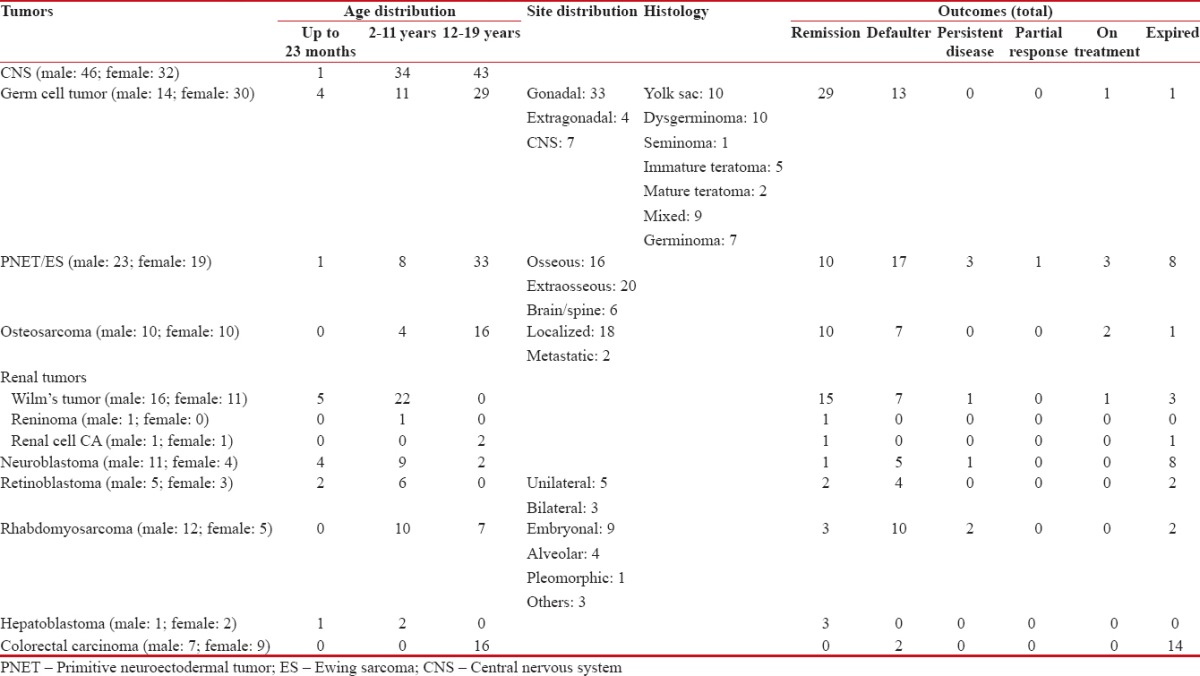
|
- Germ cell tumors (n-44): These were more frequent in females (68%) and adolescents (66%). The most common histologies were yolk sac tumors (23%) and dysgerminoma (23%) followed by mixed germ cell tumor (20%), teratoma (immature 11%, mature 5%), and seminoma (2.2%). According to site, 75%-were gonadal, 9%-were extra-gonadal, and 16%-were in CNS/spine (germinomas). Most patients were diagnosed in Stage I/III (41%- each) then Stage IV (16%) and Stage II (2.2%). Most of the patients achieved remission (66%) but 30%-defaulted and 2.2%-expired
- PNET/ES (n-42): These were more frequent in males (55%) and in adolescents (79%). Localized tumors were marginally more frequent (52.8%) as compared to metastatic disease (47.2%). According to site, 48%-were extraosseous, 38%-osseous, and 14%-in CNS/spine. Most patients defaulted (40%), remission was documented in 24%, stable disease in 7%, partial response in 2%-and 19%-expired
- Wilms' tumor (n-27): These constituted 90%-of renal tumors. They were more common in males (59.2%) and mostly presented in children in the age group of 2–11 years (81.4%). Most of the tumors were diagnosed in Stage III (44.4%), followed by Stage I (29.6%), stage IV (22.2%), and Stage II (3.7%). Most patients went into remission (55.5%), 25.9%-defaulted, 3.7%-had stable disease, and 11.1%-expired
- Osteosarcoma (n-20): These were equally prevalent in males and females. Most were diagnosed in adolescents (80%) and in localized stage (90%). Most patients went into remission (50%), but 35%-defaulted, 10%-were still on treatment, and 5%-expired
- Rhabdomyosarcoma (n-17): They were more common in males (71%) and in children in the age group of 2–11 years (59%) followed by adolescents (41%) and none in infants up to 23 months. The most common histology was embryonal (53%), followed by alveolar (24%), and pleomorphic (6%) and others (18%). Most of the tumors were diagnosed in Stage IV (47%) followed by Stage I (29%), Stage II/III (12%-each). Most patients defaulted (59%), 18%-went into remission, 12%-had stable disease, and 12%-expired
- Colorectal carcinoma (n-16): These were more frequent in females (56%), and all were adolescents (100%). Most patients were diagnosed in Stage III (50%) or Stage IV (44%) followed by Stage II (6%) and none in Stage I. Most of the patients expired (88%), and the rest (13%) defaulted
- Neuroblastoma (n-15): They were more common in males (73%) and mostly presented in children in the age group of 2–11 years (60%) followed by infants up to 23 months (27%) and children in 12–19 years age group (13%). Most of the tumors were diagnosed in Stage IV (73%), followed by Stage III (20%), stage I (7%), and none in Stage II. Most patients expired (53%), 33%-defaulted, only 7%-had remission, and 7%-had stable disease
- Retinoblastoma (n-8): They were more common in males (62%) and mostly presented in children in the age group of 2–11 years (75%) followed by infants up to 23 months (25%) and none in the 12–19 years age group. Among these, 63% were unilateral and 38%-bilateral. Most of the tumors were diagnosed in Reese-Ellsworth Group II (38%), followed by Group III (25%), Group IV (25%), and Group I (13%). Most patients defaulted (50%), 25% went into remission and 25%-expired
- Hepatoblastoma (n-3): These were more frequent in females (67%) and in children in 2–11 years age group (67%) followed by infants up to 23 months (33%)-and none among the adolescents. Most patients were diagnosed in Stage I (67%), followed by Stage IV (33%), and none in Stage II/III. All the patients achieved remission (100%)
- Benign tumors (n-17): These were rare cases – fibromatosis (n-1, remission), giant cell tumor of bone (n-2, 1 remission, 1 stable disease), inflammatory myofibromatosis (n-2, remission), meningioma (n-1, defaulted), Langerhans cell histiocytosis (n-7, 3 remissions, 4 defaulters), schwannoma (n-3, 2 defaulters, 1 stable disease) and vascular hamartoma (n-1, defaulter).
Discussion
Malignancy is the second most common cause of childhood death in the developed world, accounting for 10%–12.3%-of all childhood deaths.[11] Although child health continues to be a priority health issue in India, childhood cancer is not yet a major area of focus. Appropriate management of pediatric tumors requires complete epidemiological data of pediatric tumors in different geographical areas. In developing countries, as significant progress is made in treating infectious diseases and nutritional deficiencies, cancer is emerging as a major childhood killer.[12,13]
In India, cancer is the 9th common cause for the deaths among children between 5 and 14 years of age.[14] The proportion of childhood cancers relative to all cancers reported by Indian cancer registries varied from 0.8%-to 5.8%-in boys and from 0.5% to 3.4%-in girls.[15] There are few studies reporting childhood cancer incidence from cancer registries in Indian states. A recent publication by Satyanarayana et al. provides an updated summary overview of the incidence of childhood cancer on the basis of the 2013 report from the National Cancer Registry Program for the years 2006–2011 that covered 25 population-based cancer registries in India.[16] In low- and middle-income countries, where 80%-of children live, the 200,000 children diagnosed with cancer each year have limited access to curative treatment and only about 25%-survive.[17] The difference in survival for children diagnosed with cancer between high- and low-income countries continues to widen as curative therapies are developed in the former but not implemented in the latter.[18]
Overall, cancer in childhood is more common among males than females, and the male-to-female ratio in the most resource-rich countries is around 1.2:1.[3,19] However, some cancers such as retinoblastoma, Wilms' tumor, osteosarcoma, and germ cell tumor show a slight female preponderance. The reported incidence of childhood cancer in India in males (39–150 per million children per year) is higher than in females (23–97 per million children per year) in all population-based cancer registries (PBCRs) except in North East India, and this gives a male-to-female ratio that is much higher than what is seen in the developed world.
Outcomes approaching similar to international standards have been achieved in India in those treated at tertiary institutes like the Tata Memorial Hospital in Mumbai as per the available literature.[20] However, one cannot extrapolate these results to the whole population as often those who abandon treatment or are lost to follow-up are excluded from the analysis of hospital case series, and such patients may have a more advanced disease and a poorer outlook. PBCR survival data are a better representation of cancer outcomes across India and have been reported from Bangalore and Chennai where the 5-year overall survival for all childhood cancers combined is 37-40%. The highest survival is seen for Wilms' tumor and Hodgkins' lymphoma where approximately two-third of the children survive for 5 years or more. The survival for retinoblastoma and germ cell tumors, which are cancers with excellent prognosis in the developed world is disappointingly low and may be related to an advanced stage at presentation and suboptimal chemotherapy regimens used.[21,22] The prognosis for leukemia and CNS tumors are also low where approximately only 33%-and 25%-of the children survive at 5 years.
Pediatric solid tumors in Kashmir: Available data
One of the earliest reports regarding the pediatric solid tumors in Kashmir was published by Shah A in 1992.[23] This study did a retrospective analysis of 93 cases of childhood tumors (except leukemias) received in the Department of pathology during January 1983 to June 1989. These 93 cases formed 0.1% of total hospital admissions, 4.3% of pediatric admissions and 1.7% of all malignant tumors. Lymphoma was the most common tumor (30%), followed by Wilm's (14%), nervous system tumors, and soft tissue sarcomas (11.8%) each. There was an overall male preponderance (M: F 1.9:1), with individual tumors being: lymphoma (3.8:1), Wilm's tumor (5.5:1), neuroblastoma (2:1), and soft tissue sarcoma (1.2:1). Children below 5 years were more affected (39.8%), followed by prepubertal children and children between 6 and 9 years of age (29%). Wilm's tumor and neuroblastoma were more in children below 5 years of age whereas lymphoma and nervous system tumors were more in children above 5 years of age. The overall incidence of childhood tumors was 5.4%-similar to what was observed in Bombay (now Mumbai) at that time.
Another study was undertaken by Aziz et al. in 1986 to determine the profile of abdominal tumors in pediatric population.[24] The profile of 40 patients was studied, with majority being in the age group 4–6 years (M:F 28:12). The ratio of urban to rural background in these children was 19:21. Wilm's tumor accounted for the majority of these children, followed by non-Hodgkin's lymphoma, and Neuroblastoma. 93.33%-of Wilm's tumor patients were operable at the time of diagnosis although some residual disease was occasionally left after surgery. Only 16.66%-of neuroblastomas were operable the time of diagnosis. Surgical debulking was possible only in 41.66%-patients of non-Hodgkin's lymphoma.
A clinical study of primary abdominal tumors in children revealed that the distribution of various primary abdominal solid tumors as Wilms tumor 37.5%, Neuroblastoma 15%, Lymphomas 32.5%, Hepatocellular Carcinoma 2.5%, and others (including Teratocarcinoma stomach) 12.5%.[25]
Our data were collected from RCC, SKIMS, Srinagar, over duration from December 2008 to June 2014 and the overall incidence and prevalence of pediatric solid tumors was consistent with data from other national and international series. The incidence of pediatric cancer including solid tumors in Jammu and Kashmir has shown a steady increase over the years. It is not clear whether this is an actual rise or an increase in the proportion of patients seeking health care. Among the registered patients, around 5%-were of pediatric age group, and of these, around 30%-had solid tumors. The most common solid tumors were CNS tumors, one-fourth of the patients.
Some differences have also emerged. The male-to-female ratio was lower than that reported in the West and India. The incidence of patients is much higher from rural areas in Kashmir. Majority of the patients are adolescents (12–19 years) as compared to international data where most of the pediatric patients are in the 0–4 year age group. Overall, outcomes were worse than other series. Majority of patients were defaulters (35.64%), only 33.9%-patients achieved remission, and 20.79%-patients expired over the study duration. It is clear that the patients in Jammu and Kashmir present in an advanced stage and have worse outcomes.
The distribution of major solid tumors is also different in this region. After CNS tumors, the second most common tumor is germ cell tumor followed by PNET/ES, Wilms' tumor, osteosarcoma, rhabdomyosarcoma, colorectal cancer, neuroblastoma, and retinoblastoma. We have achieved good outcomes in hepatoblastoma, germ cell tumors, osteosarcoma, and Wilms' tumor. The rest of the patients' had poor outcomes, especially neuroblastoma and colorectal cancers.
Conclusions
This is a pioneering study of the patterns of epidemiology, pathology, and outcomes of treatment of pediatric patients with solid tumors in Jammu and Kashmir. The patients in our series are different in that a high percentage presents in an advanced stage, a lot of them default and have poor follow-up. Our study has limitations in that it is a single institution retroprospective study and has not presented survival data as that would require further prolonged follow-up. This is an area of active research in our institution.
Multiple interrelated factors are responsible for the poorer outlook of childhood cancer in India. Limited financial resources, lack of awareness of the meaning of symptoms, and difficulty in accessing healthcare, abandonment of treatment, and of course, belief in alternative medicines, contribute to advanced stage presentation. It is imperative to address these issues to improve the outcomes of our patients. This can be done by improving the levels of education and public health awareness in the state. Accessibility to health services, especially in adverse weather conditions, however, still remains a challenge. Furthermore, pediatric patients should be treated in dedicated and specialized centers as proved by the excellent results achieved in the USA and Europe, where >90%-of children are treated in such centers. Finally, advances in diagnostics including the turn around time and modalities of treatments need to be implemented to improve the outcomes of our patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Barr R, Riberio R, Agarwal B, Masera G, Hesseling P, Magrath I. Pediatric oncology in countries with limited resources. In: Pizzo PA, Poplack DG, editors. Principles and Practice of Pediatric Oncology. 5th ed. Philadelphia: Lippincott Williams and Wilkins; 2006. p. 1605-17.
- Ries LA, Smith MA, Gurney JG, Linet M, Tamra T, Young JL, et al., editors. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975-1995, National Cancer Institute, SEER Program. NIH Pub. No. 99-4649. Bethesda, MD, 1999.
- Kroll ME, Stiller CA. Time Trends in Incidence 1966-2000. Childhood Cancers in Britain: Incidence, Survival, Mortality. Oxford Scholarship Online; 2009.
- Harif M, Barsaoui S, Benchekroun S, Bouhas R, Doumbé P, Khattab M, et al. Treatment of B-cell lymphoma with LMB modified protocols in Africa – Report of the French-African Pediatric Oncology Group (GFAOP). Pediatr Blood Cancer 2008;50:1138-42.
- Howard SC, Pedrosa M, Lins M, Pedrosa A, Pui CH, Ribeiro RC, et al. Establishment of a pediatric oncology program and outcomes of childhood acute lymphoblastic leukemia in a resource-poor area. JAMA 2004;291:2471-5.
- Qaddoumi I, Musharbash A, Elayyan M, Mansour A, Al-Hussaini M, Drake J, et al. Closing the survival gap: Implementation of medulloblastoma protocols in a low-income country through a twinning program. Int J Cancer 2008;122:1203-6.
- Rivera GK, Quintana J, Villarroel M, Santana VM, Rodriguez-Galindo C, Neel MD, et al. Transfer ofcomplex frontline anticancer therapy to a developing country: The St. Jude osteosarcoma experience in Chile. Pediatr Blood Cancer 2008;50:1143-6.
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin 2009;59:225-49.
- Bleyer A, O'Leary M, Barr R, Ries LA, editors. Cancer Epidemiology in Older Adolescents and Young Adults 15 to 29 Years of Age, Including SEER Incidence and Survival: 1975-2000. NIH Publication. No. 06-5767. Bethesda, MD: National Cancer Institute; 2006.
- ;Crist WM, Anderson JR, Meza JL, Fryer C, Raney RB, Ruymann FB, et al. Intergroup rhabdomyosarcoma study-IV: Results for patients with nonmetastatic disease. J Clin Oncol 2001;19:3091-102.
- Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, et al., editors. SEER Cancer Statistics Review, 1975-2011. Bethesda, MD: National Cancer Institute. Based on November 2013 SEER Data Submission, Posted to the SEER. Available from: http://www.seer.cancer.gov/csr/1975_2011/. [Last accessed on 2014 Apr 12].
- Rathi AK, Kumar S, Ashu A, Singh K, Bahadur A. Epidemiology of pediatric tumors at a tertiary care centre. Indian J Med Paediatr Oncol 2007;28:33-5.
- Kusumakumary P, Jacob R, Jothirmayi R, Nair MK. Profile of pediatric malignancies: A ten year study. Indian Pediatr 2000;37:1234-8.
- Summary-Report on Causes of Death: 2001-2003 in India. Available from: http://www.censusindia.gov.in/Vital_Statistics/Summary_Report_Death_01_03.pdf. [Last accessed on 2013 Sep 24].
- Three Year Report of the Population Based Cancer Registries 2009-2011: Report of 25 PBCRs; National Cancer Registry Programme, Indian Council Medical Research, Bangalore; 2013. Available from: http://www.ncrpindia.org/Reports/PBCR_2009_2011.aspx. [Last accessed on 2013 Sep 24].
- Satyanarayana L, Asthana S, Labani SP. Childhood cancer incidence in India: A review of population-based cancer registries. Indian Pediatr 2014;51:218-20.
- Kellie SJ, Howard SC. Global child health priorities: What role for paediatric oncologists? Eur J Cancer 2008;44:2388-96.
- Howard SC, Marinoni M, Castillo L, Bonilla M, Tognoni G, Luna-Fineman S, et al. Improving outcomes for children with cancer in low-income countries in Latin America: A report on the recent meetings of the Monza International School of Pediatric Hematology/Oncology (MISPHO)-Part I. Pediatr Blood Cancer 2007;48:364-9.
- Gurney JG, Bondy ML. Epidemiology of childhood cancer. In: Pizzo PA, Poplack DG, editors. Principles and Practice of Paediatric Oncology. 5th ed. Philadelphia: Lippincott Williams and Wilkins; 2006. p. 2-14.
- Arora B, Kurkure P, Parikh P. Childhood cancers: Perspectives in India. J Indian Med Assoc 2005;103:479-82.
- Sahu S, Banavali SD, Pai SK, Nair CN, Kurkure PA, Motwani SA, et al. Retinoblastoma: Problems and perspectives from India. Pediatr Hematol Oncol 1998;15:501-8.
- Nair R, Pai SK, Saikia TK, Nair CN, Kurkure PA, Gopal R, et al. Malignant germ cell tumors in childhood. J Surg Oncol 1994;56:186-90.
- Shah A. Pattern of pediatric solid malignant tumors in Kashmir. Indian Pediatr 1992;29:1045-6.
- Aziz AS, Shah AH, Sheikh KA. Clinical profile of abdominal tumours in children in Kashmir. 1986. Available from: http://medind.nic.in/haa/t02/i1/haat02i1p25g.pdf. [Last accessed on 2014 Apr 17].
- ;Khan PS, Akhter Z, Majeed S, Wani MY, Hayat H. Clinicopathological Profile of Childhood Primary Abdominal Tumours in Kashmir. Indian J Surg 2015;77 Suppl 2:361-4.

| Figure 1:Age distribution

| Figure 2:Major tumor distribution
References
- Barr R, Riberio R, Agarwal B, Masera G, Hesseling P, Magrath I. Pediatric oncology in countries with limited resources. In: Pizzo PA, Poplack DG, editors. Principles and Practice of Pediatric Oncology. 5th ed. Philadelphia: Lippincott Williams and Wilkins; 2006. p. 1605-17.
- Ries LA, Smith MA, Gurney JG, Linet M, Tamra T, Young JL, et al., editors. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975-1995, National Cancer Institute, SEER Program. NIH Pub. No. 99-4649. Bethesda, MD, 1999.
- Kroll ME, Stiller CA. Time Trends in Incidence 1966-2000. Childhood Cancers in Britain: Incidence, Survival, Mortality. Oxford Scholarship Online; 2009.
- Harif M, Barsaoui S, Benchekroun S, Bouhas R, Doumbé P, Khattab M, et al. Treatment of B-cell lymphoma with LMB modified protocols in Africa – Report of the French-African Pediatric Oncology Group (GFAOP). Pediatr Blood Cancer 2008;50:1138-42.
- Howard SC, Pedrosa M, Lins M, Pedrosa A, Pui CH, Ribeiro RC, et al. Establishment of a pediatric oncology program and outcomes of childhood acute lymphoblastic leukemia in a resource-poor area. JAMA 2004;291:2471-5.
- Qaddoumi I, Musharbash A, Elayyan M, Mansour A, Al-Hussaini M, Drake J, et al. Closing the survival gap: Implementation of medulloblastoma protocols in a low-income country through a twinning program. Int J Cancer 2008;122:1203-6.
- Rivera GK, Quintana J, Villarroel M, Santana VM, Rodriguez-Galindo C, Neel MD, et al. Transfer ofcomplex frontline anticancer therapy to a developing country: The St. Jude osteosarcoma experience in Chile. Pediatr Blood Cancer 2008;50:1143-6.
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin 2009;59:225-49.
- Bleyer A, O'Leary M, Barr R, Ries LA, editors. Cancer Epidemiology in Older Adolescents and Young Adults 15 to 29 Years of Age, Including SEER Incidence and Survival: 1975-2000. NIH Publication. No. 06-5767. Bethesda, MD: National Cancer Institute; 2006.
- ;Crist WM, Anderson JR, Meza JL, Fryer C, Raney RB, Ruymann FB, et al. Intergroup rhabdomyosarcoma study-IV: Results for patients with nonmetastatic disease. J Clin Oncol 2001;19:3091-102.
- Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, et al., editors. SEER Cancer Statistics Review, 1975-2011. Bethesda, MD: National Cancer Institute. Based on November 2013 SEER Data Submission, Posted to the SEER. Available from: http://www.seer.cancer.gov/csr/1975_2011/. [Last accessed on 2014 Apr 12].
- Rathi AK, Kumar S, Ashu A, Singh K, Bahadur A. Epidemiology of pediatric tumors at a tertiary care centre. Indian J Med Paediatr Oncol 2007;28:33-5.
- Kusumakumary P, Jacob R, Jothirmayi R, Nair MK. Profile of pediatric malignancies: A ten year study. Indian Pediatr 2000;37:1234-8.
- Summary-Report on Causes of Death: 2001-2003 in India. Available from: http://www.censusindia.gov.in/Vital_Statistics/Summary_Report_Death_01_03.pdf. [Last accessed on 2013 Sep 24].
- Three Year Report of the Population Based Cancer Registries 2009-2011: Report of 25 PBCRs; National Cancer Registry Programme, Indian Council Medical Research, Bangalore; 2013. Available from: http://www.ncrpindia.org/Reports/PBCR_2009_2011.aspx. [Last accessed on 2013 Sep 24].
- Satyanarayana L, Asthana S, Labani SP. Childhood cancer incidence in India: A review of population-based cancer registries. Indian Pediatr 2014;51:218-20.
- Kellie SJ, Howard SC. Global child health priorities: What role for paediatric oncologists? Eur J Cancer 2008;44:2388-96.
- Howard SC, Marinoni M, Castillo L, Bonilla M, Tognoni G, Luna-Fineman S, et al. Improving outcomes for children with cancer in low-income countries in Latin America: A report on the recent meetings of the Monza International School of Pediatric Hematology/Oncology (MISPHO)-Part I. Pediatr Blood Cancer 2007;48:364-9.
- Gurney JG, Bondy ML. Epidemiology of childhood cancer. In: Pizzo PA, Poplack DG, editors. Principles and Practice of Paediatric Oncology. 5th ed. Philadelphia: Lippincott Williams and Wilkins; 2006. p. 2-14.
- Arora B, Kurkure P, Parikh P. Childhood cancers: Perspectives in India. J Indian Med Assoc 2005;103:479-82.
- Sahu S, Banavali SD, Pai SK, Nair CN, Kurkure PA, Motwani SA, et al. Retinoblastoma: Problems and perspectives from India. Pediatr Hematol Oncol 1998;15:501-8.
- Nair R, Pai SK, Saikia TK, Nair CN, Kurkure PA, Gopal R, et al. Malignant germ cell tumors in childhood. J Surg Oncol 1994;56:186-90.
- Shah A. Pattern of pediatric solid malignant tumors in Kashmir. Indian Pediatr 1992;29:1045-6.
- Aziz AS, Shah AH, Sheikh KA. Clinical profile of abdominal tumours in children in Kashmir. 1986. Available from: http://medind.nic.in/haa/t02/i1/haat02i1p25g.pdf. [Last accessed on 2014 Apr 17].
- ;Khan PS, Akhter Z, Majeed S, Wani MY, Hayat H. Clinicopathological Profile of Childhood Primary Abdominal Tumours in Kashmir. Indian J Surg 2015;77 Suppl 2:361-4.


 PDF
PDF  Views
Views  Share
Share

