A Prospective Single-Arm Study of Melphalan, Prednisolone and Lenalidomide (MPL) as First Line Treatment in Elderly Patients with Multiple Myeloma – An Institutional Study
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2022; 43(05): 409-414
DOI: DOI: 10.1055/s-0042-1748802
Abstract
Introduction Multiple myeloma in the elderly population is rising in India. Such frail transplant-ineligible patients are less frequently included in clinical trials. Moreover, novel agents are not accessible to everyone. Melphalan-based chemotherapy regimens are frequently used in elderly myeloma patients. Our study revisited the role of melphalan, prednisone, and lenalidomide (MPL) as front-line therapy in this subgroup of patients.
Objective The aim of this study was to determine the response, tolerance, and outcome of MPL in elderly patients with newly diagnosed multiple myeloma.
Materials and Methods This prospective study was conducted at the Department of Medical Oncology at a tertiary cancer center during January 2012 to September 2013. Newly diagnosed patients with multiple myeloma >60 years who were transplant ineligible formed the study subjects. Eligible patients received oral melphalan 0.18 mg/kg from D1 to 4, prednisone 2 mg/kg from D1 to 4, and lenalidomide 10 mg from D1 to 21 q28 days. Patients who achieved complete response/very good partial response (CR/VGPR) after 6 cycles of MPL received maintenance with lenalidomide 10 mg from D1 to 21 q28 days (MPL-L) until progression or 1 year whichever was earlier. Quality of life was assessed using the Eq. 5D questionnaire.
Results Out of 46 patients, 25 were males and 21 were females. Median age was 67 years (range: 60—83 years). Majority had immunoglobulin G myeloma, followed by immunoglobulin A subtype. The median quality of life score at baseline was 50 (range: 30–70). Forty patients completed six cycles of MPL. The main toxicity was grade 1 to 2 hematological. There were no treatment-related deaths. Twenty-two (55%) achieved CR, 5 (13%) achieved VGPR, 4 (10%) achieved partial response, 6 (15%) achieved stable disease, and 3 (7%) had progressive disease. Twenty-seven patients received lenalidomide maintenance. At a median follow-up of 55 months, the 2- and 5-year progression-free survival was 60 and 18%, respectively. The overall survival at 2 and 5 years were 80 and 53%, respectively. The median number of subsequent lines of treatment was 2 (range: 1–4). The quality of life was improved and preserved in all study subjects. At 8 years, three patients had second malignant neoplasms and seven are alive.
Conclusion MPL-L is a well-tolerated and effective regimen in elderly myeloma with good overall response rates.
Publication History
Article published online:
20 October 2022
© 2022. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Introduction Multiple myeloma in the elderly population is rising in India. Such frail transplant-ineligible patients are less frequently included in clinical trials. Moreover, novel agents are not accessible to everyone. Melphalan-based chemotherapy regimens are frequently used in elderly myeloma patients. Our study revisited the role of melphalan, prednisone, and lenalidomide (MPL) as front-line therapy in this subgroup of patients.
Objective The aim of this study was to determine the response, tolerance, and outcome of MPL in elderly patients with newly diagnosed multiple myeloma.
Materials and Methods This prospective study was conducted at the Department of Medical Oncology at a tertiary cancer center during January 2012 to September 2013. Newly diagnosed patients with multiple myeloma >60 years who were transplant ineligible formed the study subjects. Eligible patients received oral melphalan 0.18 mg/kg from D1 to 4, prednisone 2 mg/kg from D1 to 4, and lenalidomide 10 mg from D1 to 21 q28 days. Patients who achieved complete response/very good partial response (CR/VGPR) after 6 cycles of MPL received maintenance with lenalidomide 10 mg from D1 to 21 q28 days (MPL-L) until progression or 1 year whichever was earlier. Quality of life was assessed using the Eq. 5D questionnaire.
Results Out of 46 patients, 25 were males and 21 were females. Median age was 67 years (range: 60—83 years). Majority had immunoglobulin G myeloma, followed by immunoglobulin A subtype. The median quality of life score at baseline was 50 (range: 30–70). Forty patients completed six cycles of MPL. The main toxicity was grade 1 to 2 hematological. There were no treatment-related deaths. Twenty-two (55%) achieved CR, 5 (13%) achieved VGPR, 4 (10%) achieved partial response, 6 (15%) achieved stable disease, and 3 (7%) had progressive disease. Twenty-seven patients received lenalidomide maintenance. At a median follow-up of 55 months, the 2- and 5-year progression-free survival was 60 and 18%, respectively. The overall survival at 2 and 5 years were 80 and 53%, respectively. The median number of subsequent lines of treatment was 2 (range: 1–4). The quality of life was improved and preserved in all study subjects. At 8 years, three patients had second malignant neoplasms and seven are alive.
Conclusion MPL-L is a well-tolerated and effective regimen in elderly myeloma with good overall response rates.
Introduction
Multiple myeloma (MM) is a plasma cell disorder that accounts for 10 to 15% of hematological malignancies. The incidence of MM in India is lower when compared with the West (1/1,00,000 vs 4.1/1,00,000), but, as per recent statistics, the numbers are expanding.[1] As the life expectancy of the general population is on the rise, the proportion of elderly patients with various malignancies is expected to rise as well. MM deserves special mention in this seam, as the majority affected are elderly, frail, and transplant ineligible. The median survival of elderly patients with MM has improved in recent years with the advent of newer agents, starting from bortezomib in late 2000s.[1] In 1990s, combination of melphalan, prednisone, and thalidomide was considered as a standard of care in elderly MM, who were transplant ineligible.[1] Present study looked at the role of lenalidomide along with melphalan and prednisone, herein after referred as MPL-L (melphalan-prednisone-lenalidomide induction followed by lenalidomide maintenance) in elderly patients as front-line therapy in MM.
Materials and Methods
This prospective single-arm study was conducted at the Department of Medical Oncology at our tertiary cancer Institute, in India, during 2012 to 2013. Patients with newly diagnosed MM above 60 years of age, transplant ineligible, were included in the study. Patients with an Eastern Co-operative Oncology Group performance status of ≤2 with adequate organ function as indicated by the laboratory tests (hemoglobin [Hb] > 8 g/dL, platelet count >75000/mm3, absolute neutrophil count > 1000/mm3, serum creatinine <2 href="https://www.thieme-connect.com/products/ejournals/html/10.1055/s-0042-1748802#JR213740556-2" xss=removed>2] Response to treatment was assessed by International Myeloma Working Group criteria.[3] Toxicity was graded using CTCAE v3. Quality of life (QoL) was assessed using the Eq. 5D questionnaire.[4] The toxicity assessment was done at each visit. The QoL was assessed at baseline, after 3 and 6 months of MPL and after 12 months of maintenance lenalidomide. The primary endpoint was response assessment, that is, attainment of CR/VGPR/PR)/SD/progressive disease (PD), and the secondary endpoints were progression-free survival (PFS), overall survival (OS), toxicity assessment, and QoL. During maintenance, clinical examination and laboratory investigations (Hb, total WBC count, platelet count, serum creatinine, blood urea, serum calcium, immunoglobulin assay, free light chain assay, and serum electrophoresis) were done q3monthly. Further follow-up was done q6monthly with clinical examination and investigations, as above, or earlier if the patient was symptomatic. PFS was calculated from the date of diagnosis to the date of first progression or death. OS was calculated from the date of diagnosis to the date of last follow-up or death.
Statistical Analysis
The categorical variables are expressed in frequency and proportion. The continuous variables are summarized using mean and standard deviation. The OS and PFS are estimated using Kaplan–Meier method and association of survival with comparator parameters is tested using logrank test. The risk is estimated using Cox Regression. A p-value of <0>
Ethics
The study was approved by the Human Ethics Committee (HEC 37/2011, dated January 12, 2012). The trial is registered under the Clinical Trials Registry - India (CTRI) (CTRI/2013/04/003565). Written informed consent was obtained from all study subjects. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Results
Baseline Characteristics
A total of 65 patients were screened for the study. Out of 55 patients who were eligible for the study, 46 patients received the study drug. Nine patients did not report back for treatment. Twenty-five patients were males and 21 were females. The median age was 67 years (range: 60–83 years). Seventeen (37%) patients were in 60 to 65 years age group, 14 (30%) were in 66 to 70 years, and 15 (33%) patients were in >70 years age group. Twenty patients had documented comorbidities at the time of study entry. Thirty-three patients had IgG myeloma, 7 had immunoglobulin A (IgA) myeloma, 5 had light chain myeloma, and 1 had nonsecretory myeloma. According to the ISS staging system, 19 had stage I, 13 had stage II, and 14 had stage III MM. All had CRAB (hypercalcemia, renal impairment, anemia and bone lesions) criteria at presentation. Seven patients had hypercalcemia, 10 patients had renal impairment, 15 patients had anemia, and 40 patients had bone lesions. Urine Bence-Jones proteins were demonstrable in six patients. The median QoL score at baseline was 50 (range: 30–70).
Treatment Received and Outcome
All 46 patients received at least one cycle of chemotherapy and 40 patients completed six cycles of MPL. The main toxicity was grade 1 to 2 hematological in nature. Nonhematological toxicities included fatigue (grade 1–2), pneumonitis (grade 1), and deep vein thrombosis (grade 2). Four (8.7%) patients required granulocyte colony stimulating factors (GCSF) and two (4.3%) patients had febrile neutropenia that were managed conservatively. Two patients were withdrawn from the study, the reasons being allergy to lenalidomide and severe hematological toxicity to chemotherapy. There were no treatment-related deaths. The summary of adverse events is shown in [Table 1].
|
Grade 1 n (%) |
Grade 2 n (%) |
Grade 3 n (%) |
Grade 4 n (%) |
|
|---|---|---|---|---|
|
Hematological toxicity |
||||
|
Anemia |
17 (37) |
17 (37) |
4 (9) |
1 (2) |
|
Thrombocytopenia |
17 (37) |
6 (13) |
6 (13) |
1 (2) |
|
Neutropenia |
14 (30) |
12 (26) |
3 (6) |
1 (2) |
|
2 (4.3%) patients developed febrile neutropenia |
||||
|
Nonhematological toxicity |
||||
|
Fatigue |
15 (33) |
10 (22) |
0 |
0 |
|
Pneumonitis |
4 (9) |
0 |
0 |
0 |
|
Deep vein thrombosis |
0 |
1 (2) |
0 |
0 |
|
Rash |
4 |
0 |
0 |
0 |
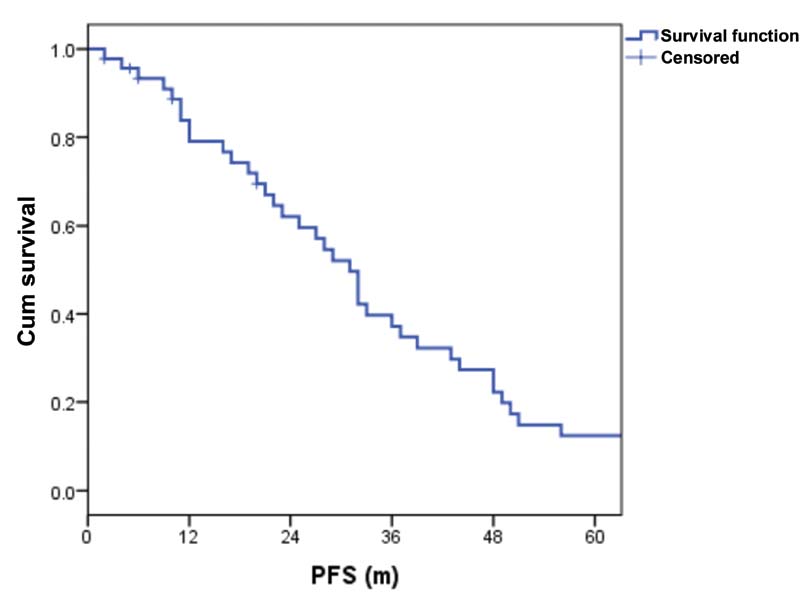
| Figure 1:Progression-free survival (PFS).
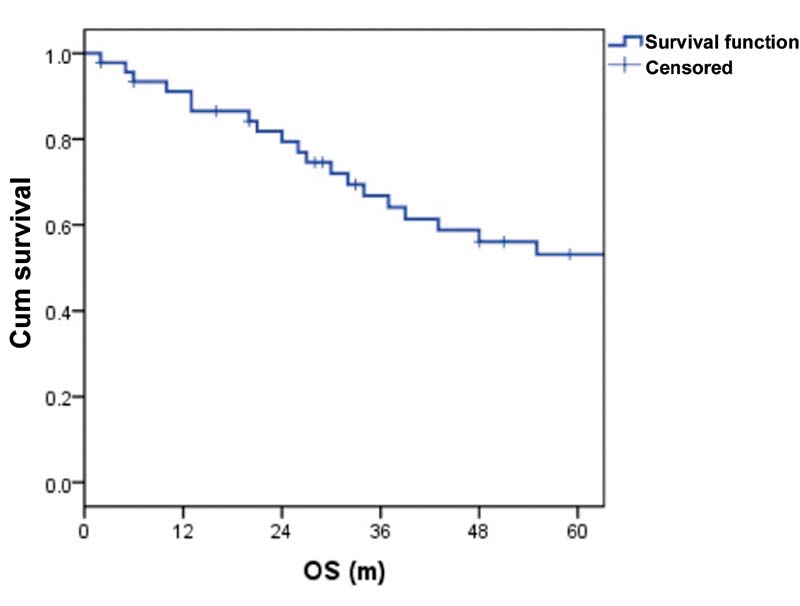
| Figure 2:Overall survival (OS).
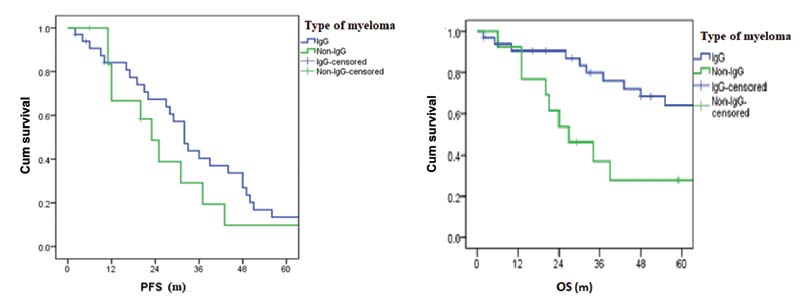
| Figure 3:(A) Progression-free survival (PFS) versus type of myeloma. (B) Overall survival versus type of myeloma (OS). IgG, immunoglobulin G.
Twenty-four patients received next line of treatment, with bortezomib-based chemotherapy in the majority, at a median duration of 31 months (range: 8–102 months). The median number of subsequent lines of treatment was 2 (range: 1–4). The median PFS in those who completed 1 year maintenance was 41 months (range: 20–111 months) and median OS was 69 months (range: 20–111 months). At 8-year follow-up, seven patients are alive ([Fig. 4]). Three developed second malignancies, in the form of squamous cell carcinoma esophagus at 2 years, clear cell renal cell carcinoma at 3 years, and carcinoma larynx at 5 years.
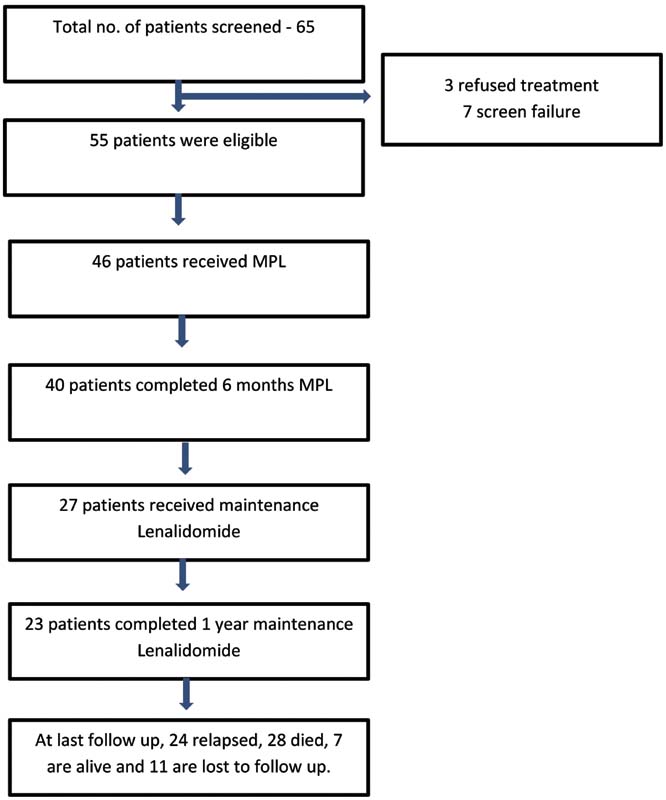
| Figure 4:Consolidated Standards of Reporting Trials (CONSORT) diagram. MPL, melphalan, prednisone, and lenalidomide.
QoL Assessment
The QoL was improved and preserved in all the study subjects. At the point of study entry, the median QoL was 50 (range: 30–70). The median QoL after 3 cycles of MPL, 6 cycles of MPL, and after 12 cycles of maintenance lenalidomide were 60 (50–90), 90 (50–100), and 100, respectively.
Discussion
The treatment armamentarium of elderly patients with MM is abounding. Choosing the upfront treatment of an elderly transplant ineligible MM patient depends on the performance status, organ reserve, comorbidities, and polypharmacy. MP, the once considered standard of care in elderly MM has undergone various combinations and has evolved to the present treatment schedules. The added benefit of thalidomide to MP (MPT) was demonstrated in the benchmark trial, Intergroupe Francophone du Myelome (IFM) 99–06, and henceforth was continued as the new standard of care in the treatment of elderly MM in late 1990s and early 2000s.[5] MPT was better than MP in terms of PFS, OS, and time to progression. The benefit of lenalidomide in combination with MP was studied by Palumbo et al in 2007 and it was found to be an effective regimen with good response rates.[6] A subsequent phase I/II trial of MPL in transplant ineligible MM patients, with a median age of 74 years, demonstrated an objective response rate of 69% and manageable toxicity profile.[7] In the phase III trial EA106, Stewart et al compared MPT and MPL in newly diagnosed transplant ineligible MM. In this study, both the study arms had similar PFS and OS with a favorable hematological (73 vs. 58%; p = 0.007) and nonhematological (59 vs. 40%; p =0.001) toxicity profile for MPL.[8] Palumbo et al in 2012 investigated the role of MPL induction followed by maintenance lenalidomide (MPL-L) among 459 transplant ineligible MM patients. MPL-L was found to significantly improve the PFS, and the greatest benefit was seen in 65 to 75 years age group.[9]
Our study investigated the role of MPL followed by maintenance lenalidomide as front-line in transplant ineligible MM patients. In our study, IgG subtype and ISS stage 1 were the commonest. The regimen was well tolerable and all the described toxicities were managed conservatively. There were 16 grade 3 to 4 hematological events. Two (4.3%) patients had febrile neutropenia and four (8.7%) required GCSF support. There was no treatment-related mortality. However, in the MM-015 study, grade 3 to 4 hematological toxicities are reported as the most common.[9]
In our study, the percentage of patients with at least a PR was 78%-after six MPL. As per MM-015 study also, a CR/PR rate of 77%-was seen in the MPL-L regimen. The median PFS in MM-015 study was better for MPL-L than MP, 31 and 13 months, respectively, with continued benefit at maintenance of lenalidomide as well.[9]
As the majority of clinical trials enroll fit and young patients, the data on elderly patients is very scarce. Focus on the tolerability and QoL maintenance is imperative in managing elderly MM patients. As per MM-015 study, 16%-of patients had to discontinue the drug due to adverse events; however, it is lower than that with MPT (33–45%) and V(bortezomib) MP (12–34%). In our study, only one patient was withdrawn due to poor treatment tolerance. In EA106 trial, which compared melphalan, prednisone, thalidomide with thalidomide maintenance (MPT-T) and melphalan-prednisone-lenalidomide with lenalidomide maintenance (MPR-R), survival was similar in both, while QoL assessment favored MPR-R arm at the end of induction.[10] In our study group, the QoL was maintained and was progressively better throughout the treatment protocol unless the disease was progressive. The 2- and 5-year PFS were 60 and 18%, respectively, and the OS at 2 and 5 years were 80 and 53%, respectively. The survival was better for IgG subtype in our study. ISS was not statistically significant for PFS in our study, probably due to the small sample size and as the majority were in ISS stage 1. The median number of subsequent lines of treatment was 2 (range: 1–4). At 8-year follow-up, seven patients are alive and three developed second malignancies.
Our study has the limitation of a small sample size. We could not perform baseline cytogenetics in our study as it was unavailable during the study period. Elderly, frail patients with MM who are not fit for intensive chemotherapy can have meaningful remission rates with MPL-L regimen. This is a well-tolerated and effective oral regimen and studies comparing this with bortezomib-based regimens in elderly MM need to be explored in the future.
Conclusion
To conclude, MPL-L is an effective and well-tolerated regimen in elderly myeloma with good overall response rates.
Disclaimer
Verified that all authors have read and approved the manuscript and there is no conflict of interest or any financial disclosures. Agree that the manuscript work is original, has not been submitted, or is under review for publication elsewhere.
Conflict of Interest
None declared.
Acknowledgment
None.
References
- Kumar L, Nair S, Vadlamani SP, Chaudhary P. Multiple myeloma: an update. J Curr Oncol 2020; 3: 72-80
- Greipp PR, San Miguel J, Durie BG. et al. International Staging System for Multiple Myeloma. J Clin Oncol 2005; 23 (15) 3412-3420
- Rajkumar SV, Dimopoulos MA, Palumbo A. et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 2014; 15 (12) e538-e548
- Preedy VR, Watson RR. EQ-5D. Handbook of Disease Burdens and Quality of Life Measures. 2010. Springer; NY: https://doi.org/10.1007/978-0-387-78665-0_5607
- Facon T, Mary JY, Hulin C. et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99–06): a randomised trial. Lancet 2007; ; 6;370(9594): 1209-1218 DOI: 10.1016/S0140-6736(07)61537-2.
- Palumbo A, Falco P, Corradini P. et al; GIMEMA–Italian Multiple Myeloma Network. Melphalan, prednisone, and lenalidomide treatment for newly diagnosed myeloma: a report from the GIMEMA–Italian Multiple Myeloma Network. J Clin Oncol 2007; 25 (28) 4459-4465
- Roy V, Stewart A, Bergsagel P. et al. Phase II study of melphalan, prednisone and lenalidomide combination for newly diagnosed multiple myeloma patients who are not candidates for stem cell transplantation [abstract]. Blood. 2008 ; 112 Abstract 2769.
- Stewart A, Jacobus S, Fonseca F. et al. E1A06: A phase III trial comparing melphalan, prednisone, and thalidomide (MPT) versus melphalan, prednisone, and lenalidomide (MPR) in newly diagnosed multiple myeloma (MM) [Abstract]. J Clin Oncol.. 2014 ; 32 (5 s_suppl) Abstract 8511.
- Palumbo A, Hajek R, Delforge M. et al; MM-015 Investigators. Continuous lenalidomide treatment for newly diagnosed multiple myeloma. N Engl J Med 2012; 366 (19) 1759-1769
- Stewart AK, Jacobus S, Fonseca R. et al. Melphalan, prednisone, and thalidomide vs melphalan, prednisone, and lenalidomide (ECOG E1A06) in untreated multiple myeloma. Blood 2015; 126 (11) 1294-1301
Address for correspondence
Geetha Narayanan, MD, DMDepartment of Medical Oncology, Regional Cancer CentreTrivandrum 695011, KeralaIndiaEmail: geenarayanan@yahoo.comPublication History
Article published online:
20 October 2022© 2022. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Figure 1:Progression-free survival (PFS).

| Figure 2:Overall survival (OS).

| Figure 3:(A) Progression-free survival (PFS) versus type of myeloma. (B) Overall survival versus type of myeloma (OS). IgG, immunoglobulin G.

| Figure 4:Consolidated Standards of Reporting Trials (CONSORT) diagram. MPL, melphalan, prednisone, and lenalidomide.
References
- Kumar L, Nair S, Vadlamani SP, Chaudhary P. Multiple myeloma: an update. J Curr Oncol 2020; 3: 72-80
- Greipp PR, San Miguel J, Durie BG. et al. International Staging System for Multiple Myeloma. J Clin Oncol 2005; 23 (15) 3412-3420
- Rajkumar SV, Dimopoulos MA, Palumbo A. et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 2014; 15 (12) e538-e548
- Preedy VR, Watson RR. EQ-5D. Handbook of Disease Burdens and Quality of Life Measures. 2010. Springer; NY: https://doi.org/10.1007/978-0-387-78665-0_5607
- Facon T, Mary JY, Hulin C. et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99–06): a randomised trial. Lancet 2007; ; 6;370(9594): 1209-1218 DOI: 10.1016/S0140-6736(07)61537-2.
- Palumbo A, Falco P, Corradini P. et al; GIMEMA–Italian Multiple Myeloma Network. Melphalan, prednisone, and lenalidomide treatment for newly diagnosed myeloma: a report from the GIMEMA–Italian Multiple Myeloma Network. J Clin Oncol 2007; 25 (28) 4459-4465
- Roy V, Stewart A, Bergsagel P. et al. Phase II study of melphalan, prednisone and lenalidomide combination for newly diagnosed multiple myeloma patients who are not candidates for stem cell transplantation [abstract]. Blood. 2008 ; 112 Abstract 2769.
- Stewart A, Jacobus S, Fonseca F. et al. E1A06: A phase III trial comparing melphalan, prednisone, and thalidomide (MPT) versus melphalan, prednisone, and lenalidomide (MPR) in newly diagnosed multiple myeloma (MM) [Abstract]. J Clin Oncol.. 2014 ; 32 (5 s_suppl) Abstract 8511.
- Palumbo A, Hajek R, Delforge M. et al; MM-015 Investigators. Continuous lenalidomide treatment for newly diagnosed multiple myeloma. N Engl J Med 2012; 366 (19) 1759-1769
- Stewart AK, Jacobus S, Fonseca R. et al. Melphalan, prednisone, and thalidomide vs melphalan, prednisone, and lenalidomide (ECOG E1A06) in untreated multiple myeloma. Blood 2015; 126 (11) 1294-1301


 PDF
PDF  Views
Views  Share
Share

