Analysis of Relapsed/Refractory Hodgkin Lymphoma Treated with Autologous Transplantation: A Single-Center Experience
CC BY-NC-ND 4.0 ? Indian J Med Paediatr Oncol 2020; 41(01): 23-28
DOI: DOI: 10.4103/ijmpo.ijmpo_64_19
Abstract
Introduction:?Hodgkin lymphoma (HL) is one of the common lymphomas with high cure rate.?Aims:?The aim was to study the outcome of relapsed/refractory HL treated with autologous transplantation.?Objectives:?The objective was to study the overall survival, overall response, and disease-free survival of the relapsed/refractory HL after autologous transplantation.?Methods:?It was a retrospective study conducted over a period of 8 years in our center using computer-based database and medical records as the data source.?Results:?A total of 22 patients were diagnosed with relapsed/refractory HL of which majority of cases were male patients (59%) with a mean age of 29 years (range: 15?57 years) and were Stage 4A (40.9%), with nodular sclerosis (54.5%). The overall response rate was 81.8% with 9.1% complete response, 72.7% partial response, and 4.5% stable disease; the overall survival was 77.92 ? 6.65 months, and disease-free survival was 69.66 ? 8.13 months.?Conclusion:?Autologous stem cell transplant plays an integral role in the treatment of patients with relapsed/refractory Hodgkin lymphoma.
 ?
?Keywords
Autologous - Hodgkin - refractory - relapsedPublication History
Received: 06 March 2019
Accepted: 24 November 2019
Publication Date:
23 May 2021 (online)
? 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Introduction:?Hodgkin lymphoma (HL) is one of the common lymphomas with high cure rate.?Aims:?The aim was to study the outcome of relapsed/refractory HL treated with autologous transplantation.?Objectives:?The objective was to study the overall survival, overall response, and disease-free survival of the relapsed/refractory HL after autologous transplantation.?Methods:?It was a retrospective study conducted over a period of 8 years in our center using computer-based database and medical records as the data source.?Results:?A total of 22 patients were diagnosed with relapsed/refractory HL of which majority of cases were male patients (59%) with a mean age of 29 years (range: 15?57 years) and were Stage 4A (40.9%), with nodular sclerosis (54.5%). The overall response rate was 81.8% with 9.1% complete response, 72.7% partial response, and 4.5% stable disease; the overall survival was 77.92 ? 6.65 months, and disease-free survival was 69.66 ? 8.13 months.?Conclusion:?Autologous stem cell transplant plays an integral role in the treatment of patients with relapsed/refractory Hodgkin lymphoma.
Keywords
Autologous - Hodgkin - refractory - relapsedIntroduction
Hodgkin lymphoma (HL) is a type of malignancy with high cure rates. However, about 15% of patients with early-stage disease and about 20%?30% with advanced-stage disease either have refractory disease or tend to have relapse of the disease after initial therapy.[1] Approximately 80% of patients have symptoms or signs of the disease at relapse.[2] The goal of the treatment in relapsed/refractory HL is long-term disease control while minimizing the toxicity and complications of therapy. High-dose chemotherapy followed by autologous stem cell transplant (ASCT) is the treatment of choice for relapsed/refractory disease.[3] [4] Although ASCT has not shown to improve the overall survival, it is considered the standard of care.
Methods
This was a retrospective study conducted from January 2010 to July 2018 in the department of hematology at our 200-bedded tertiary cancer hospital. The study was initiated using computer-based database and medical records as the data source. Relapsed/refractory HL cases were defined as clinically symptomatic or progressive or PET-CT scan relapsed/progressive patients with or without proven histopathology. Relapse case was defined as a case that came back after the remission from first-line chemotherapy. The refractory case was defined as a case that progressed during the treatment with first-line chemotherapy.
A study pro forma was established, which included patient identification, diagnosis, staging, histological types, international prognostic index score, chemotherapy types and number of cycles, date of relapse, conditioning regimen, and date of transplant. Patient?s disease status till the last follow-up was recorded along with the date of relapse and/or death. After the collection of reports, statistical analysis was conducted using SPSS Software version 20. The primary objective of our study was to calculate the overall survival post autologous transplant, while the secondary objectives were to calculate the overall response and disease-free survival in our patients. Survival curves were plotted with Kaplan?Meier methodology. Patients who were lost to follow-up after therapy were censored as alive at their last follow-up. Descriptive statistics was used for the analysis of demographic variables, disease characteristics, and response rates.
Results
Patients characteristics
A total of 22 patients were diagnosed with relapsed/refractory HL during the study period. In our study, majority of the cases were male patients (59%) with a mean age of 29 years (range 15?57 years) and were Stage 4A (40.9%), with nodular sclerosis (54.5%) and International Prognostic Score (IPS) score of two (40.9%). B symptoms at diagnosis were recorded in 45% of the patients. None of the patients were diagnosed with tuberculosis prior to the histological diagnosis of HL ? one of the differential diagnoses in these patients. Nine percent of the patients had comorbidities. All (100%) of the patients had the Eastern Cooperative Oncology Group performance status (ECOG-PS) of ?2. None of the patients had acquired immunodeficiency syndrome at the time of diagnosis, and no patients had a history of exposure to radiation before the diagnosis [Table 1].
Table 1Clinical characteristics of patients
|
Patients characteristics |
n (%) |
|---|---|
|
TB ? Tuberculosis; ECOG ? Eastern Cooperative Oncology Group |
|
|
Mean age |
29.6?12 |
|
Male |
13 (59.1) |
|
Female |
9 (40.9) |
|
Age group |
|
|
15?30 |
16 (73) |
|
30?45 |
3 (13.5) |
|
45?60 |
3 (13.5) |
|
>60 |
0 |
|
National patients |
17 (77.5) |
|
International patients |
5 (22.5) |
|
Professional |
11 (50) |
|
Students |
7 (32) |
|
Homemaker |
4 (18) |
|
Comorbidity |
2 (9) |
|
TB |
0 |
|
Retropositivity |
0 |
|
Radiation exposure before |
0 |
|
ECOG 1 |
22 (100) |
Lymphoma characteristics
Stage 4A was the most common presentation with 40.9%. The most common histopathology was nodular sclerosis observed in 54.5%, followed by mixed cellularity, lymphocyte-predominant type, and lymphocyte-depleted type, in 36.5%, 4.5%, and 4.5%, respectively. IPS could be estimated in all the patients. IPS score ?4 was observed in 95.5% (21/22) of the patients. No bone marrow involvement and extranodal sites of disease was observed. Bulky disease (defined as largest tumor size ?10 cm) was also not observed in our patients. There were 9 relapsed cases and 13 refractory cases in total [Table 2].
Table 2Clinical characteristics of lymphoma
|
Lymphoma characteristics |
n (%) |
|---|---|
|
HL ? Hodgkin lymphoma; IPS ? International prognostic score |
|
|
Staging |
|
|
IA |
1 (4.5) |
|
IB |
1 (4.5) |
|
IIB |
3 (13.6) |
|
IIIA |
2 (9.1) |
|
IIIB |
3 (13.6) |
|
IVA |
9 (40.9) |
|
IVB |
3 (13.6) |
|
Histological types |
|
|
Lymphocyte depleted |
1 (4.5) |
|
Mixed cellularity |
8 (36.5) |
|
Nodular lymphocyte predominent HL |
1 (4.5) |
|
Nodular sclerosis |
12 (54.5) |
|
IPS score |
|
|
1 |
8 (36.4) |
|
2 |
9 (40.9) |
|
3 |
4 (18.2) |
|
4 |
0 |
|
5 |
1 (4.5) |
|
B symptoms |
10 (45) |
|
Relapsed cases |
9 (41.5) |
|
Refractory cases |
13 (58.5) |
Treatment characteristics
Most of the patients (n?= 21 patients, 95.5%) were treated with standard ABVD chemoregimen before the relapse, while 4.5% of the patients received CHOP regimen due to advanced age or comorbidities. Six cycles of chemotherapy were completed by all of the patients. Radiation was given to 22.5% of the patients for either bulky sites or residual disease at the end of chemotherapy. Post relapsed/refractory, the most common second-line regimen used was ifosfamide, carboplatin, and etoposide (ICE) regimen (55%), followed by dexamethasone, high-dose Ara-C, and Cisplatin (DHAP); gemcitabine, dexamethasone, and cisplatin; and mesna, ifosfamide, mitoxantrone, and etoposide regimens. Majority of the patients after the second-line chemotherapy had partial response (PR) (73%) before undergoing autologous transplantation. Post autologous transplant, four patients had relapsed, of which two patients received the third-line six cycles of Gemox chemotherapy, while one patient received radiation to the relapsed mediastinum. About 40.5% of the patients developed an infection during the transplant, which was salvageable with broad-spectrum antibiotics. Transplant-related mortality was zero during our transplantation [Table 3].
Table 3 Treatment characteristics
Transplantation characteristics
All the relapsed/refractory patients underwent autologous transplantation at our center. The conditioning regimen used was BEAM regimen (100%) to treat patients for autologous transplantation. The mean stem cell collection during the stem cell transplantation was 6.022 ? 3.4 ? 10 ? 6/kg and the mean neutrophil engraftment duration was 13.3 ? 3.8 days. The overall response rate was 81.8% with 9.1% complete response (CR), 72.7% PR, and 4.5% stable disease. The overall survival was 77.92 ? 6.65 months and disease-free survival was 69.66 ? 8.13 months as shown in [Figures 1] and [2], respectively. There were two deaths (9.0%) during the follow-up period, one with the relapse and other with acute myeloid leukemia transformation. A total of four re-relapses occurred after autologous transplantation, and six (27%) patients were lost to follow-up [Table 4].
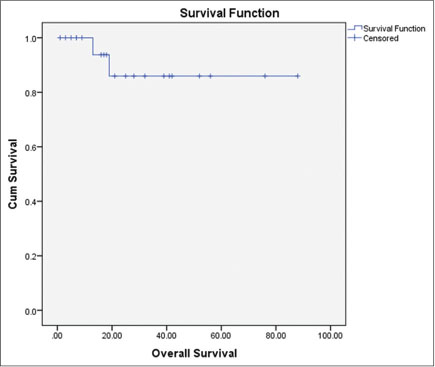
|?Fig. 1: Overall survival postautologous transplantation
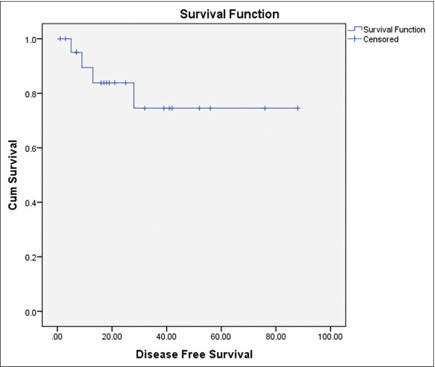
|?Fig. 2: Disease free survival post autologous transplantation
Table 4 Transplantation characteristics
Discussion
This report comprises about 96-month follow-up of a single institution cohort of patients with refractory/relapsed HL who underwent ASCT. The results showed that high-dose chemotherapy followed by ASCT can induce disease control and improve the prognosis of patients with advanced refractory or relapsed HL. The patient population of this study was heterogeneous and pretreated, with a median of two lines of chemotherapy regimens before transplantation. Despite this, 91% of the patients were alive and 86.5% disease-free at the end of the follow-up.
The diagnosis of relapsed or refractory HL is mostly done clinically as most patients are symptomatic at presentation. Occasionally, however, new lesions or lesions at the initial site are picked up on imaging studies. NCCN v1.2017 recommends obtaining contrast computed tomography (CT) scan of the neck, thorax, abdomen, and pelvis at 6, 12, and 24 months after completion of the therapy or as clinically indicated. The role of positron-emission tomography (PET)-CT scan in the diagnosis of relapsed or refractory disease is still not clearly defined. NCCN v1.2017 recommends PET-CT only if the last PET was Deauville 4?5 to confirm CR. Although these imaging modalities expose the patient to various risks, the benefits over clinical detection are not very clear.[5] [6] Repeat biopsies of the persistent or new lesions are recommended. Repeat biopsies are especially useful in patients with refractory disease and late relapses (3?5 years after primary treatment) or treating physician suspects another malignancy. In our study, clinical examination and PET-CT scan with or without biopsy had been used to confirm the relapsed/refractory cases.
Several prognostic factors have been evaluated by various investigators to predict survival in relapsed/refractory disease.[7] [8] [9] [10] [11] [12] These include patient-related factors such as poor PS, age >50 years, the presence of B symptoms, poor response to the first chemotherapy, low albumin, anemia, and lymphocytopenia. Disease-related factors include Stage III/IV at presentation, extranodal disease, relapse in the previous radiation field, residual disease pretransplant, and time to relapse <1>
Several studies have emphasized the need for cytoreduction prior to transplant. Hence, various salvage chemotherapy regimens have been studied in randomized controlled trials.[9] [13] [14] [15] [16] [17] [18] [19] [20] [21] [22] However, there is no gold standard salvage chemotherapy, as head-to-head comparison among these various regimens has not been conducted. Several regimens such as gemcitabine, vinorelbine, and pegylated liposomal doxorubicin; ifosfamide, gemcitabine, and vinorelbine; and gemcitabine, carboplatin, and dexamethasone have also been tried with good results.[23] [24] [25] However, these regimens have not been tried in randomized controlled trials. The decision to use the appropriate regimen is usually made by the treating physician according to the institutional experience and protocols and individual patient-related factors. One of the major factors which decide on the choice of salvage chemotherapy regimens is the impact on subsequent peripheral blood stem cell (PBSC) mobilization. Regimens containing melphalan have been reported to impair PBSC mobilization.[26] [27] Hence, regimens which do not contain melphalan like ICE and DHAP have become popular in recent years. One study concluded that although both ICE regimen and DHAP regimens were safe, the former had slightly more response rates.[28] Bendamustine, lenalidomide, and everolimus have also been tried in several Phase 2 trials. We have used ICE regimen in majority of cases and we have found the maximum benefits with minimum side effects.
Newer class of agents have also been used in Phase 2 trials. Notable among these are the antibody?drug conjugates and programmed death receptor-1 (PD-1) inhibitors (immunotherapy). Brentuximab vedotin (anti-CD30 antibody?drug conjugate) was initially used as single agent. Recently, there has been an attempt to combine this agent with conventional salvage chemotherapy regimens with good success rates.[29] [30] [31] [32] [33] [34] [35] Nivolumab (PD-1 inhibitor) has also been used recently. A Phase 1/2 trial demonstrated good efficacy and safety when combined with brentuximab.[36] Further larger Phase 3 studies are required to validate the results of these preliminary studies involving newer agents. BEAM regimen is most commonly used as high-dose conditioning chemotherapy before ASCT. A trial by GHSG reported higher freedom from treatment failure in patients who underwent ASCT following HDC compared to HDC alone. The overall survival however remained the same.[37] Hence, NCCN v12017 recommends HDC followed by ASCT for all patients with relapsed/refractory HL.
Survival after relapse post-ASCT remains poor. It is estimated that up to 50% of patients ultimately relapse following ASCT. Treatment of such patients is particularly challenging. RIC allogenic SCT remains one of the options but is limited by various factors such as donor availability, patient preference, and lack of sensitivity of the disease to chemotherapy. In our study, a total of four cases relapsed and one patient developed acute myeloid leukemia post autologous transplantation. Of four patients, one died, two received third-line GEMOX chemotherapy, and fourth received radiation to the mediastinum. Later, three patients were doing well till the last follow-up.
Brentuximab vedotin has been successfully used in such patients. The 5-year survival rates are encouraging considering the heavily pretreated nature of these individuals. It can be used even if treated previously in the same patient.[38] [39] [40] Nivolumab and pembrolizumab have also been used in relapse post-ASCT with good results.[41] [42] [43] Very recently, a combination of immunotherapy and brentuximab was administered in a small group of patients who had relapsed post-ASCT. The overall response rates were 100%. No serious Grade 3/4 toxicities which would lead to treatment discontinuation were observed.[44] Lenalidomide alone or in combination with bendamustine has been tried. Other agents which have given mixed results are histone deacetylase inhibitors such as panobinostat and mTOR inhibitor everolimus.[45] [46] [47] However, we did not use any of these newer treatments in our re-relapsed cases.
The strength of this study lies in the fact that this is one of the considerable number of patients? data from India on relapsed/refractory HL treated with autologous transplantation. However, this is a retrospective analysis which has its inherent flaws of missing data and lack of follow-up.
Conclusion
ASCT plays an integral role in the treatment of patients with HL. Most patients with HL are cured with combination chemotherapy with or without radiation therapy. ASCT is currently the optimal treatment for patients who fail chemotherapy and radiation therapy. Treatment of relapsed HL post autologous transplantation is particularly challenging.
Consent
Informed consent was obtained from all patients for being included in the study.
Conflict of Interest
There are no conflicts of interest.
References
- ?Bonadonna G, Viviani S, Bonfante V, Gianni AM, Valagussa P.?Survival in Hodgkin?s disease patients-report of 25 years of experience at the Milan Cancer Institute. Eur J Cancer 2005; 41: 998-1006
- Radford JA, Eardley A, Woodman C, Crowther D.?Follow up policy after treatment for Hodgkin?s disease: Too many clinic visits and routine tests? A review of hospital records. BMJ 1997; 314: 343-6
- Lazarus HM, Rowlings PA, Zhang MJ, Vose JM, Armitage JO, Bierman PJ. et al.?Autotransplants for Hodgkin?s disease in patients never achieving remission: A report from the Autologous Blood and Marrow Transplant Registry. J Clin Oncol 1999; 17: 534-45
- Andr? M, Henry-Amar M, Pico JL, Brice P, Blaise D, Kuentz M. et al.?Comparison of high-dose therapy and autologous stem-cell transplantation with conventional therapy for Hodgkin?s disease induction failure: A case-control study. Soci?t? Francaise de Greffe de Moelle. J Clin Oncol 1999; 17: 222-9
- Goldschmidt N, Or O, Klein M, Savitsky B, Paltiel O.?The role of routine imaging procedures in the detection of relapse of patients with Hodgkin lymphoma and aggressive non-Hodgkin lymphoma. Ann Hematol 2011; 90: 165-71
- Krause SW, Gerken M, Andreesen R, Hofst?dter F, Klinkhammer-Schalke M.?Treatment of B cell lymphoma with chemotherapy plus rituximab: A survival benefit can be demonstrated in the routine data of a regional cancer registry. Ann Hematol 2012; 91: 561-70
- Zinzani PL, Stefoni V, Tani M, Fanti S, Musuraca G, Castellucci P. et al.?Role of [18F] fluorodeoxyglucose positron emission tomography scan in the follow-up of lymphoma. J Clin Oncol 2009; 27: 1781-7
- Brice P, Bouabdallah R, Moreau P, Divine M, Andr? M, Aoudjane M. et al.?Prognostic factors for survival after high-dose therapy and autologous stem cell transplantation for patients with relapsing Hodgkin?s disease: Analysis of 280 patients from the French registry. Soci?t? Fran?aise de Greffe de Mo?lle. Bone Marrow Transplant 1997; 20: 21-6
- Moskowitz CH, Nimer SD, Zelenetz AD, Trippett T, Hedrick EE, Filippa DA. et al.?A 2-step comprehensive high-dose chemoradiotherapy second-line program for relapsed and refractory Hodgkin disease: Analysis by intent to treat and development of a prognostic model. Blood 2001; 97: 616-23
- ?Josting A, Franklin J, May M, Koch P, Beykirch MK, Heinz J. et al.?New prognostic score based on treatment outcome of patients with relapsed Hodgkin?s lymphoma registered in the database of the German Hodgkin?s lymphoma study group. J Clin Oncol 2002; 20: 221-30
- Sureda A, Constans M, Iriondo A, Arranz R, Caballero MD, Vidal MJ. et al.?Prognostic factors affecting long-term outcome after stem cell transplantation in Hodgkin?s lymphoma autografted after a first relapse. Ann Oncol 2005; 16: 625-33
- Horning SJ, Chao NJ, Negrin RS, Hoppe RT, Long GD, Hu WW. et al.?High-dose therapy and autologous hematopoietic progenitor cell transplantation for recurrent or refractory Hodgkin?s disease: Analysis of the Stanford University results and prognostic indices. Blood 1997; 89: 801-13
- Jabbour E, Hosing C, Ayers G, Nunez R, Anderlini P, Pro B. et al.?Pretransplant positive positron emission tomography/gallium scans predict poor outcome in patients with recurrent/refractory Hodgkin lymphoma. Cancer 2007; 109: 2481-9
- Pfreundschuh MG, Rueffer U, Lathan B, Schmitz N, Brosteanu O, Hasenclever D. et al.?Dexa-BEAM in patients with Hodgkin?s disease refractory to multidrug chemotherapy regimens: A trial of the German Hodgkin?s Disease Study Group. J Clin Oncol 1994; 12: 580-6
- Colwill R, Crump M, Couture F, Danish R, Stewart AK, Sutton DM. et al.?Mini-BEAM as salvage therapy for relapsed or refractory Hodgkin?s disease before intensive therapy and autologous bone marrow transplantation. J Clin Oncol 1995; 13: 396-402
- Rodriguez J, Rodriguez MA, Fayad L, McLaughlin P, Swan F, Sarris A. et al.?ASHAP: A regimen for cytoreduction of refractory or recurrent Hodgkin?s disease. Blood 1999; 93: 3632-6
- Baetz T, Belch A, Couban S, Imrie K, Yau J, Myers R. et al.?Gemcitabine, dexamethasone and cisplatin is an active and non-toxic chemotherapy regimen in relapsed or refractory Hodgkin?s disease: A phase II study by the National Cancer Institute of Canada Clinical Trials Group. Ann Oncol 2003; 14: 1762-7
- Chau I, Harries M, Cunningham D, Hill M, Ross PJ, Archer CD. et al.?Gemcitabine, cisplatin and methylprednisolone chemotherapy (GEM-P) is an effective regimen in patients with poor prognostic primary progressive or multiply relapsed Hodgkin?s and non-Hodgkin?s lymphoma. Br J Haematol 2003; 120: 970-7
- Ferm? C, Mounier N, Divin? M, Brice P, Stamatoullas A, Reman O. et al.?Intensive salvage therapy with high-dose chemotherapy for patients with advanced Hodgkin?s disease in relapse or failure after initial chemotherapy: Results of the Groupe d?Etudes des Lymphomes de l?Adulte H89 Trial. J Clin Oncol 2002; 20: 467-75
- Hertzberg MS, Crombie C, Benson W, Taper J, Gottlieb D, Bradstock KF.?Outpatient-based ifosfamide, carboplatin and etoposide (ICE) chemotherapy in transplant-eligible patients with non-Hodgkin?s lymphoma and Hodgkin?s disease. Ann Oncol 2003; 14 (01) Suppl i11-6
- Josting A, Rudolph C, Reiser M, Mapara M, Sieber M, Kirchner HH. et al.?Time-intensified dexamethasone/cisplatin/cytarabine: An effective salvage therapy with low toxicity in patients with relapsed and refractory Hodgkin?s disease. Ann Oncol 2002; 13: 1628-35
- Aparicio J, Segura A, Garcer? S, Oltra A, Santaballa A, Yuste A. et al.?ESHAP is an active regimen for relapsing Hodgkin?s disease. Ann Oncol 1999; 10: 593-5
- Bartlett NL, Niedzwiecki D, Johnson JL, Friedberg JW, Johnson KB, van Besien K. et al.?Gemcitabine, vinorelbine, and pegylated liposomal doxorubicin (GVD), a salvage regimen in relapsed Hodgkin?s lymphoma: CALGB 59804. Ann Oncol 2007; 18: 1071-9
- Crump M, Kuruvilla J, Couban S, MacDonald DA, Kukreti V, Kouroukis CT. et al.?Randomized comparison of gemcitabine, dexamethasone, and cisplatin versus dexamethasone, cytarabine, and cisplatin chemotherapy before autologous stem-cell transplantation for relapsed and refractory aggressive lymphomas: NCIC-CTG LY.12. J Clin Oncol 2014; 32: 3490-6
- Santoro A, Magagnoli M, Spina M, Pinotti G, Siracusano L, Michieli M. et al.?Ifosfamide, gemcitabine, and vinorelbine: A new induction regimen for refractory and relapsed Hodgkin?s lymphoma. Haematologica 2007; 92: 35-41
- Dreger P, Kl?ss M, Petersen B, Haferlach T, L?ffler H, Loeffler M. et al.?Autologous progenitor cell transplantation: Prior exposure to stem cell-toxic drugs determines yield and engraftment of peripheral blood progenitor cell but not of bone marrow grafts. Blood 1995; 86: 3970-8
- Weaver CH, Zhen B, Buckner CD.?Treatment of patients with malignant lymphoma with Mini-BEAM reduces the yield of CD34+ peripheral blood stem cells. Bone Marrow Transplant 1998; 21: 1169-70
- Abali H, Ur?n Y, Oks?zo?lu B, Budako?lu B, Yildirim N, G?ler T. et al.?Comparison of ICE (ifosfamide-carboplatin-etoposide) versus DHAP (cytosine arabinoside-cisplatin-dexamethasone) as salvage chemotherapy in patients with relapsed or refractory lymphoma. Cancer Invest 2008; 26: 401-6
- Chen R, Palmer JM, Martin P, Tsai N, Kim Y, Chen BT. et al.?Results of a multicenter phase II trial of brentuximab vedotin as second-line therapy before autologous transplantation in relapsed/refractory Hodgkin lymphoma. Biol Blood Marrow Transplant 2015; 21: 2136-40
- Chen R, Palmer J, Martin P, Armenian S, Tsai N, Mott M. et al.?Post transplant outcomes in a multicenter phase II study of brentuximab vedotin as first line salvage therapy in relapsed or refractory Hodgkin lymphoma prior to autologous stem cell transplantation. Haematologica 2016; 101: 47-8
- Moskowitz AJ, Sch?der H, Yahalom J, McCall SJ, Fox SY, Gerecitano J. et al.?PET-adapted sequential salvage therapy with brentuximab vedotin followed by augmented ifosamide, carboplatin, and etoposide for patients with relapsed and refractory Hodgkin?s lymphoma: A non-randomised, open-label, single-centre, phase 2 study. Lancet Oncol 2015; 16: 284-92
- Garcia-Sanz R, Sureda A, de la Cruz F, Canales M, Gonzalez AP, Pinana JL. et al.?Brentuximab vedotin and ESHAP is highly effective as second-line therapy for Hodgkin lymphoma patients (long-term results of a trial by the Spanish GELTAMO Group). Ann Oncol 2019; 30: 612-20
- Cassaday RD, Fromm J, Cowan AJ, Libby EN, Philip M, Behnia S. et al.?Safety and activity of brentuximab vedotin (BV) plus ifosfamide, carboplatin, and etoposide (ICE) for relapsed/refractory (Rel/Ref) classical hodgkin lymphoma (cHL): Initial results of a phase I/II study. Blood 2016; 128: 1834-44
- Hazendonk H, Fijnvandraat K, Lock J, Driessens M, van der Meer F, Meijer K. et al.?A population pharmacokinetic model for perioperative dosing of factor VIII in hemophilia A patients. Haematologica 2016; 101: 1159-69
- LaCasce AS, Bociek RG, Sawas A, Caimi P, Agura E, Matous J. et al.?Brentuximab vedotin plus bendamustine: A highly active first salvage regimen for relapsed or refractory Hodgkin lymphoma. Blood 2018; 132: 40-8
- Herrera A, Bartlett NL, Ramchandren R, Vose JM, Moskowitz A, Feldman TA. et al.?Preliminary results from a phase 1/2 study of brentuximab vedotin in combination with nivolumab in patients with relapsed or refractory Hodgkin lymphoma. Blood 2016; 128: 1105
- Schmitz N, Pfistner B, Sextro M, Sieber M, Carella AM, Haenel M. et al.?Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin?s disease: A randomised trial. Lancet 2002; 359: 2065-71
- Younes A, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ. et al.?Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin?s lymphoma. J Clin Oncol 2012; 30: 2183-9
- Chen R, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ. et al.?Five-year survival and durability results of brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma. Blood 2016; 128: 1562-6
- Bartlett NL, Chen R, Fanale MA, Brice P, Gopal A, Smith SE. et al.?Retreatment with brentuximab vedotin in patients with CD30-positive hematologic malignancies. J Hematol Oncol 2014; 7: 24
- Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M. et al.?PD-1 blockade with nivolumab in relapsed or refractory Hodgkin?s lymphoma. N Engl J Med 2015; 372: 311-9
- Younes A, Santoro A, Shipp M, Zinzani PL, Timmerman JM, Ansell S. et al.?Nivolumab for classical Hodgkin?s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: A multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol 2016; 17: 1283-94
- Armand P, Shipp MA, Ribrag V, Michot JM, Zinzani PL, Kuruvilla J. et al.?Programmed Death-1 blockade with pembrolizumab in patients with classical Hodgkin lymphoma after brentuximab vedotin failure. J Clin Oncol 2016; 34: 3733-9
- Diefenbach CS, Hong F, David KA. et al.?A phase I study with an expansion cohort of the combination of ipilimumab and nivolumab and brentuximab vedotin in patients with relapsed/refractory Hodgkin lymphoma: A trial of the ECOG-ACRIN Cancer Research Group (E4412 Arms D and E). Blood 2016; 128: 1106
- Fehniger TA, Larson S, Trinkaus K, Siegel MJ, Cashen AF, Blum KA. et al.?A phase 2 multicenter study of lenalidomide in relapsed or refractory classical Hodgkin lymphoma. Blood 2011; 118: 5119-25
- Younes A, Sureda A, Ben-Yehuda D, Zinzani PL, Ong TC, Prince HM. et al.?Panobinostat in patients with relapsed/refractory Hodgkin?s lymphoma after autologous stem-cell transplantation: Results of a phase II study. J Clin Oncol 2012; 30: 2197-203
- Johnston PB, Inwards DJ, Colgan JP, Laplant BR, Kabat BF, Habermann TM. et al.?A phase II trial of the oral mTOR inhibitor everolimus in relapsed Hodgkin lymphoma. Am J Hematol 2010; 85: 320-4
Address for correspondence
Publication History
Received: 06 March 2019
Accepted: 24 November 2019
Publication Date:
23 May 2021 (online)
? 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

|?Fig. 1: Overall survival postautologous transplantation

|?Fig. 2: Disease free survival post autologous transplantation
References
- 1?Bonadonna G, Viviani S, Bonfante V, Gianni AM, Valagussa P.?Survival in Hodgkin?s disease patients-report of 25 years of experience at the Milan Cancer Institute. Eur J Cancer 2005; 41: 998-1006
- 2?Radford JA, Eardley A, Woodman C, Crowther D.?Follow up policy after treatment for Hodgkin?s disease: Too many clinic visits and routine tests? A review of hospital records. BMJ 1997; 314: 343-6
- 3?Lazarus HM, Rowlings PA, Zhang MJ, Vose JM, Armitage JO, Bierman PJ. et al.?Autotransplants for Hodgkin?s disease in patients never achieving remission: A report from the Autologous Blood and Marrow Transplant Registry. J Clin Oncol 1999; 17: 534-45
- 4?Andr? M, Henry-Amar M, Pico JL, Brice P, Blaise D, Kuentz M. et al.?Comparison of high-dose therapy and autologous stem-cell transplantation with conventional therapy for Hodgkin?s disease induction failure: A case-control study. Soci?t? Francaise de Greffe de Moelle. J Clin Oncol 1999; 17: 222-9
- 5?Goldschmidt N, Or O, Klein M, Savitsky B, Paltiel O.?The role of routine imaging procedures in the detection of relapse of patients with Hodgkin lymphoma and aggressive non-Hodgkin lymphoma. Ann Hematol 2011; 90: 165-71
- 6?Krause SW, Gerken M, Andreesen R, Hofst?dter F, Klinkhammer-Schalke M.?Treatment of B cell lymphoma with chemotherapy plus rituximab: A survival benefit can be demonstrated in the routine data of a regional cancer registry. Ann Hematol 2012; 91: 561-70
- 7?Zinzani PL, Stefoni V, Tani M, Fanti S, Musuraca G, Castellucci P. et al.?Role of [18F] fluorodeoxyglucose positron emission tomography scan in the follow-up of lymphoma. J Clin Oncol 2009; 27: 1781-7
- 8?Brice P, Bouabdallah R, Moreau P, Divine M, Andr? M, Aoudjane M. et al.?Prognostic factors for survival after high-dose therapy and autologous stem cell transplantation for patients with relapsing Hodgkin?s disease: Analysis of 280 patients from the French registry. Soci?t? Fran?aise de Greffe de Mo?lle. Bone Marrow Transplant 1997; 20: 21-6
- 9?Moskowitz CH, Nimer SD, Zelenetz AD, Trippett T, Hedrick EE, Filippa DA. et al.?A 2-step comprehensive high-dose chemoradiotherapy second-line program for relapsed and refractory Hodgkin disease: Analysis by intent to treat and development of a prognostic model. Blood 2001; 97: 616-23
- 10?Josting A, Franklin J, May M, Koch P, Beykirch MK, Heinz J. et al.?New prognostic score based on treatment outcome of patients with relapsed Hodgkin?s lymphoma registered in the database of the German Hodgkin?s lymphoma study group. J Clin Oncol 2002; 20: 221-30
- 11?Sureda A, Constans M, Iriondo A, Arranz R, Caballero MD, Vidal MJ. et al.?Prognostic factors affecting long-term outcome after stem cell transplantation in Hodgkin?s lymphoma autografted after a first relapse. Ann Oncol 2005; 16: 625-33
- 12?Horning SJ, Chao NJ, Negrin RS, Hoppe RT, Long GD, Hu WW. et al.?High-dose therapy and autologous hematopoietic progenitor cell transplantation for recurrent or refractory Hodgkin?s disease: Analysis of the Stanford University results and prognostic indices. Blood 1997; 89: 801-13
- 13?Jabbour E, Hosing C, Ayers G, Nunez R, Anderlini P, Pro B. et al.?Pretransplant positive positron emission tomography/gallium scans predict poor outcome in patients with recurrent/refractory Hodgkin lymphoma. Cancer 2007; 109: 2481-9
- 14?Pfreundschuh MG, Rueffer U, Lathan B, Schmitz N, Brosteanu O, Hasenclever D. et al.?Dexa-BEAM in patients with Hodgkin?s disease refractory to multidrug chemotherapy regimens: A trial of the German Hodgkin?s Disease Study Group. J Clin Oncol 1994; 12: 580-6
- 15?Colwill R, Crump M, Couture F, Danish R, Stewart AK, Sutton DM. et al.?Mini-BEAM as salvage therapy for relapsed or refractory Hodgkin?s disease before intensive therapy and autologous bone marrow transplantation. J Clin Oncol 1995; 13: 396-402
- 16?Rodriguez J, Rodriguez MA, Fayad L, McLaughlin P, Swan F, Sarris A. et al.?ASHAP: A regimen for cytoreduction of refractory or recurrent Hodgkin?s disease. Blood 1999; 93: 3632-6
- 17?Baetz T, Belch A, Couban S, Imrie K, Yau J, Myers R. et al.?Gemcitabine, dexamethasone and cisplatin is an active and non-toxic chemotherapy regimen in relapsed or refractory Hodgkin?s disease: A phase II study by the National Cancer Institute of Canada Clinical Trials Group. Ann Oncol 2003; 14: 1762-7
- 18?Chau I, Harries M, Cunningham D, Hill M, Ross PJ, Archer CD. et al.?Gemcitabine, cisplatin and methylprednisolone chemotherapy (GEM-P) is an effective regimen in patients with poor prognostic primary progressive or multiply relapsed Hodgkin?s and non-Hodgkin?s lymphoma. Br J Haematol 2003; 120: 970-7
- 19?Ferm? C, Mounier N, Divin? M, Brice P, Stamatoullas A, Reman O. et al.?Intensive salvage therapy with high-dose chemotherapy for patients with advanced Hodgkin?s disease in relapse or failure after initial chemotherapy: Results of the Groupe d?Etudes des Lymphomes de l?Adulte H89 Trial. J Clin Oncol 2002; 20: 467-75
- 20?Hertzberg MS, Crombie C, Benson W, Taper J, Gottlieb D, Bradstock KF.?Outpatient-based ifosfamide, carboplatin and etoposide (ICE) chemotherapy in transplant-eligible patients with non-Hodgkin?s lymphoma and Hodgkin?s disease. Ann Oncol 2003; 14 (01) Suppl i11-6
- 21?Josting A, Rudolph C, Reiser M, Mapara M, Sieber M, Kirchner HH. et al.?Time-intensified dexamethasone/cisplatin/cytarabine: An effective salvage therapy with low toxicity in patients with relapsed and refractory Hodgkin?s disease. Ann Oncol 2002; 13: 1628-35
- 22?Aparicio J, Segura A, Garcer? S, Oltra A, Santaballa A, Yuste A. et al.?ESHAP is an active regimen for relapsing Hodgkin?s disease. Ann Oncol 1999; 10: 593-5
- 23?Bartlett NL, Niedzwiecki D, Johnson JL, Friedberg JW, Johnson KB, van Besien K. et al.?Gemcitabine, vinorelbine, and pegylated liposomal doxorubicin (GVD), a salvage regimen in relapsed Hodgkin?s lymphoma: CALGB 59804. Ann Oncol 2007; 18: 1071-9
- 24?Crump M, Kuruvilla J, Couban S, MacDonald DA, Kukreti V, Kouroukis CT. et al.?Randomized comparison of gemcitabine, dexamethasone, and cisplatin versus dexamethasone, cytarabine, and cisplatin chemotherapy before autologous stem-cell transplantation for relapsed and refractory aggressive lymphomas: NCIC-CTG LY.12. J Clin Oncol 2014; 32: 3490-6
- 25?Santoro A, Magagnoli M, Spina M, Pinotti G, Siracusano L, Michieli M. et al.?Ifosfamide, gemcitabine, and vinorelbine: A new induction regimen for refractory and relapsed Hodgkin?s lymphoma. Haematologica 2007; 92: 35-41
- 26?Dreger P, Kl?ss M, Petersen B, Haferlach T, L?ffler H, Loeffler M. et al.?Autologous progenitor cell transplantation: Prior exposure to stem cell-toxic drugs determines yield and engraftment of peripheral blood progenitor cell but not of bone marrow grafts. Blood 1995; 86: 3970-8
- 27?Weaver CH, Zhen B, Buckner CD.?Treatment of patients with malignant lymphoma with Mini-BEAM reduces the yield of CD34+ peripheral blood stem cells. Bone Marrow Transplant 1998; 21: 1169-70
- 28?Abali H, Ur?n Y, Oks?zo?lu B, Budako?lu B, Yildirim N, G?ler T. et al.?Comparison of ICE (ifosfamide-carboplatin-etoposide) versus DHAP (cytosine arabinoside-cisplatin-dexamethasone) as salvage chemotherapy in patients with relapsed or refractory lymphoma. Cancer Invest 2008; 26: 401-6
- 29?Chen R, Palmer JM, Martin P, Tsai N, Kim Y, Chen BT. et al.?Results of a multicenter phase II trial of brentuximab vedotin as second-line therapy before autologous transplantation in relapsed/refractory Hodgkin lymphoma. Biol Blood Marrow Transplant 2015; 21: 2136-40
- 30?Chen R, Palmer J, Martin P, Armenian S, Tsai N, Mott M. et al.?Post transplant outcomes in a multicenter phase II study of brentuximab vedotin as first line salvage therapy in relapsed or refractory Hodgkin lymphoma prior to autologous stem cell transplantation. Haematologica 2016; 101: 47-8
- 31?Moskowitz AJ, Sch?der H, Yahalom J, McCall SJ, Fox SY, Gerecitano J. et al.?PET-adapted sequential salvage therapy with brentuximab vedotin followed by augmented ifosamide, carboplatin, and etoposide for patients with relapsed and refractory Hodgkin?s lymphoma: A non-randomised, open-label, single-centre, phase 2 study. Lancet Oncol 2015; 16: 284-92
- 32?Garcia-Sanz R, Sureda A, de la Cruz F, Canales M, Gonzalez AP, Pinana JL. et al.?Brentuximab vedotin and ESHAP is highly effective as second-line therapy for Hodgkin lymphoma patients (long-term results of a trial by the Spanish GELTAMO Group). Ann Oncol 2019; 30: 612-20
- 33?Cassaday RD, Fromm J, Cowan AJ, Libby EN, Philip M, Behnia S. et al.?Safety and activity of brentuximab vedotin (BV) plus ifosfamide, carboplatin, and etoposide (ICE) for relapsed/refractory (Rel/Ref) classical hodgkin lymphoma (cHL): Initial results of a phase I/II study. Blood 2016; 128: 1834-44
- 34?Hazendonk H, Fijnvandraat K, Lock J, Driessens M, van der Meer F, Meijer K. et al.?A population pharmacokinetic model for perioperative dosing of factor VIII in hemophilia A patients. Haematologica 2016; 101: 1159-69
- 35?LaCasce AS, Bociek RG, Sawas A, Caimi P, Agura E, Matous J. et al.?Brentuximab vedotin plus bendamustine: A highly active first salvage regimen for relapsed or refractory Hodgkin lymphoma. Blood 2018; 132: 40-8
- 36?Herrera A, Bartlett NL, Ramchandren R, Vose JM, Moskowitz A, Feldman TA. et al.?Preliminary results from a phase 1/2 study of brentuximab vedotin in combination with nivolumab in patients with relapsed or refractory Hodgkin lymphoma. Blood 2016; 128: 1105
- 37?Schmitz N, Pfistner B, Sextro M, Sieber M, Carella AM, Haenel M. et al.?Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin?s disease: A randomised trial. Lancet 2002; 359: 2065-71
- 38?Younes A, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ. et al.?Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin?s lymphoma. J Clin Oncol 2012; 30: 2183-9
- 39?Chen R, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ. et al.?Five-year survival and durability results of brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma. Blood 2016; 128: 1562-6
- 40?Bartlett NL, Chen R, Fanale MA, Brice P, Gopal A, Smith SE. et al.?Retreatment with brentuximab vedotin in patients with CD30-positive hematologic malignancies. J Hematol Oncol 2014; 7: 24
- 41?Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M. et al.?PD-1 blockade with nivolumab in relapsed or refractory Hodgkin?s lymphoma. N Engl J Med 2015; 372: 311-9
- 42?Younes A, Santoro A, Shipp M, Zinzani PL, Timmerman JM, Ansell S. et al.?Nivolumab for classical Hodgkin?s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: A multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol 2016; 17: 1283-94
- 43?Armand P, Shipp MA, Ribrag V, Michot JM, Zinzani PL, Kuruvilla J. et al.?Programmed Death-1 blockade with pembrolizumab in patients with classical Hodgkin lymphoma after brentuximab vedotin failure. J Clin Oncol 2016; 34: 3733-9
- 44?Diefenbach CS, Hong F, David KA. et al.?A phase I study with an expansion cohort of the combination of ipilimumab and nivolumab and brentuximab vedotin in patients with relapsed/refractory Hodgkin lymphoma: A trial of the ECOG-ACRIN Cancer Research Group (E4412 Arms D and E). Blood 2016; 128: 1106
- 45?Fehniger TA, Larson S, Trinkaus K, Siegel MJ, Cashen AF, Blum KA. et al.?A phase 2 multicenter study of lenalidomide in relapsed or refractory classical Hodgkin lymphoma. Blood 2011; 118: 5119-25
- 46?Younes A, Sureda A, Ben-Yehuda D, Zinzani PL, Ong TC, Prince HM. et al.?Panobinostat in patients with relapsed/refractory Hodgkin?s lymphoma after autologous stem-cell transplantation: Results of a phase II study. J Clin Oncol 2012; 30: 2197-203
- 47?Johnston PB, Inwards DJ, Colgan JP, Laplant BR, Kabat BF, Habermann TM. et al.?A phase II trial of the oral mTOR inhibitor everolimus in relapsed Hodgkin lymphoma. Am J Hematol 2010; 85: 320-4


 PDF
PDF  Views
Views  Share
Share

