Bortezomib
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2010; 31(04): 143-144
DOI: DOI: 10.4103/0971-5851.76199

|
Publication History
Article published online:
16 August 2021
© 2010. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
INTRODUCTION
Bortezomib is a reversible inhibitor of the 26S proteasome, a protein complex that degrades ubiquitinated proteins. Proteins entering the proteasome are stripped of their ubiquitin, and subsequently degraded through catalytic activities within the core of the proteasome. The compound bortezomib (PS-341) contains a boronate moiety linked to a dipeptide and has exceedingly high affinity, specificity, and selectivity for catalytic activity of the proteasome.
Nuclear factor-κB (NF-κB) is a transcription factor that is retained in the cytoplasm when it is bound to an inhibitory partner protein, IκB. When IκB undergoes regulated serine phosphorylation, it is ubiquitinated and degraded in the proteasome. The released NF-κB moves to the nucleus, where it induces the transcription of genes whose protein products block cell-death pathways, promote cell proliferation, and regulate the expression of adhesion molecules. Inhibition of IκB degradation by proteasome inhibitors keeps NF-κB in the cytoplasm, thereby preventing it from acting on nuclear DNA.
Modification of IκB also results in the sensitization of tumor cells to chemotherapeutic agents and radiation. However, the inhibition of the proteasome has other consequences, including decreased activation of the mitogen-activated protein kinase pathway and upregulation of p53 and the cell cycle inhibitor p27 [Figure 1]
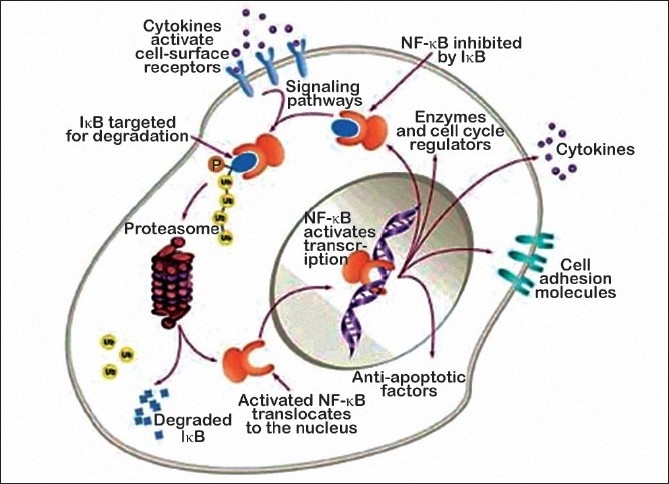
| Figure 1:Mechanism of action of bortezomib
Bortezomib has a distribution half-life of 10 minutes with wide interpatient variability in concentration. It is metabolized by oxidative deboronation via CYP 3A4 and 2C19. It has a terminal half-life of 9–15 hours.
INDICATIONS
Multiple myeloma and relapsed mantle cell lymphoma.
ADVERSE EVENTS
Peripheral neuropathy (36–37%, severe 8–14%): This is a common and often dose-limiting side effect. It is predominantly sensory, although mixed sensory–motor and autonomic neuropathy are also known to occur. Feet are affected more often than hands. The mechanism underlying bortezomib-induced peripheral neuropathy is not known. Patients with baseline symptoms are at a greater risk of developing severe neuropathy. Early detection and appropriate dosage adjustments may prevent development of severe neuropathies.
Thrombocytopenia (35–43%): Patients receiving bortezomib experience a median 60

| Figure 1:Mechanism of action of bortezomib


 PDF
PDF  Views
Views  Share
Share

