Case Report of a Glioma Patient with Homozygous Missense Amino Acid Substitution in KDR Gene
CC BY 4.0 · Indian J Med Paediatr Oncol 2023; 44(03): 356-359
DOI: DOI: 10.1055/s-0043-1762919
Abstract
Gliomas are the most commonly seen cancers of the central nervous system with a variable genetic predisposition. Here, we report a homozygous missense variant in the KDR gene in a patient with recurrent glioma. The 35-year-old male patient was diagnosed with stage IV glioma with a recurrence after 10 years from a low-grade stage two glioma. The patient underwent a repeat right craniotomy and ventriculoperitoneal shunt placement. Biopsy of the lesion showed areas of necrosis with microvascular proliferation and multinucleated tumor cells. An in-depth analysis of NGS data comprising a multigene panel of 351 genes (Agilent Cancer Core Panel) found a homozygous missense variant in exon 25 of the KDR gene that resulted in a substitution of an amino acid glutamine for arginine at codon 1118. The KDR gene or VEGF2 receptor is a type III receptor tyrosine kinase of the VEGF gene involved in angiogenesis. We hypothesize that the variation in the KDR gene may have a role in the patient's transition from grade II to grade IV glioma. While the clinical relevance of this mutation is not clear, screening mutations in the protein tyrosine and serine/threonine kinase domain of the KDR will provide critical insights into the development and progression of glioma in the pediatric and adult populations.
Keywords
glioma - Protein Kinase - KDRAuthors' Contributions
All authors have contributed and approved the manuscript.
Patient consent
The authors certify that they have obtained all appropriate patient consent forms
Statement of Ethics
This retrospective review of patient data did not require ethical approval under local guidelines. Written informed consent was obtained from the patient to publish this case report.
Publication History
Article published online:
12 May 2023
© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Gliomas are the most commonly seen cancers of the central nervous system with a variable genetic predisposition. Here, we report a homozygous missense variant in the KDR gene in a patient with recurrent glioma. The 35-year-old male patient was diagnosed with stage IV glioma with a recurrence after 10 years from a low-grade stage two glioma. The patient underwent a repeat right craniotomy and ventriculoperitoneal shunt placement. Biopsy of the lesion showed areas of necrosis with microvascular proliferation and multinucleated tumor cells. An in-depth analysis of NGS data comprising a multigene panel of 351 genes (Agilent Cancer Core Panel) found a homozygous missense variant in exon 25 of the KDR gene that resulted in a substitution of an amino acid glutamine for arginine at codon 1118. The KDR gene or VEGF2 receptor is a type III receptor tyrosine kinase of the VEGF gene involved in angiogenesis. We hypothesize that the variation in the KDR gene may have a role in the patient's transition from grade II to grade IV glioma. While the clinical relevance of this mutation is not clear, screening mutations in the protein tyrosine and serine/threonine kinase domain of the KDR will provide critical insights into the development and progression of glioma in the pediatric and adult populations.
Keywords
glioma - Protein Kinase - KDRIntroduction
Gliomas are the most frequent neoplasms of the central nervous system (CNS) originating from glial cells in older adults (mean age of 65 years). They are diffusely infiltrative tumors that affect the surrounding brain tissue. Glioblastoma multiforme is the most malignant type of glioma, while pilocytic astrocytoma is the least. Based on the histopathological analysis, gliomas are graded into four types (I–IV). The first type, Grade I glioma, is easily curable as it is usually benign. The second type, Grade II glioma, also called lower grade glioma (LGG), is often encountered in young adults. LGG is characterized by seizures and lesions in the temporal, frontal, or insular lobes. Most glioma cases detected belong to Grade III or Grade IV. In the United States, there are six cases of gliomas diagnosed per 100,000 people every year.[1] In India, 1 to 4 brain tumors per 100,000 cases occur. Glioblastoma multiforme (GBM) accounts for 18%-of all primary brain tumors and 45.9% of all glioma tumors (data from Population-based Cancer Registry, GCRI). Thus, overall, the epidemiological data on GBM tumors indicates that the incidence of this malignancy is increasing in India.[2] Genetic factors, along with environmental influence, are known to cause gliomas. LGG in a young adult may be more genetic in etiology. As more genetic studies are being done, several new genetic biomarkers are found to be associated with specific cancers. We report a case of recurrent gliomas, with increasing severity, in a young individual found to have a germline mutation in the KDR gene.
Case Report
A 35-year-old man with recurrent glioma was diagnosed with LGG in 2013, for which he underwent right-sided craniotomy and radiation therapy. This time he came in with aggressive secondary GBM, likely originating from the previous LGG. The patient presented with symptoms of generalized seizures and ECOG (Eastern Cooperative Oncology Group) performance status of level 3. Magnetic resonance imaging (MRI) brain showed right frontal craniotomy changes, including meningocele, along with new lesions in the right frontal lobe. The patient underwent a repeat right craniotomy and ventriculoperitoneal shunt placement. Biopsy of the lesion showed areas of necrosis with microvascular proliferation and multinucleated tumor cells. The patient was diagnosed as IDH mutated, ATRX mutated, GBM WHO grade IV. The patient was started on antiepileptics. He was also started on the chemotherapy agent Lomustine.
The attending physician sought tumor profiling and multigene panel testing to aid in drug decision-making of Foods and Drug Administration (FDA)-approved therapeutic molecules for approved biomarkers because the genetic factor was assumed to be linked to carcinogenesis. They investigated the correlation between non-routinely assessed oncogenes from a panel of 351 genes (Agilent Cancer Core Panel; [Supplementary Table S1], available online only>) and clinical, morphological, and molecular features to isolate gene variants, which might hold a diagnostic or prognostic significance and potential relevance for treatment. The DNA was extracted from FFPE blocks using the MN NucleoSpin DNA FFPE XS kit, followed by NGS Library preparation using SureSelect XT HS2 DNA system, a hybrid capture-based technology that includes 351 genes and subjected to paired-end sequencing on Illumina Novoseq 6000 platform. A total of 9.5 GB of raw data were generated, followed by the quality screening of raw FASTQ files, adapter trimming, mapping of raw data to the hg38 reference genome and generation of Sam/Bam files. VCF file was generated using the GATK 4.2.2 pipeline. Annotation of VCF file was using Ensembl VEP and Oncotator.
Results of Gene Panel test: Variant analysis in the targeted genes found a homozygous missense variant in exon 25 of the KDR gene (chr4:55089425C > T; NM_002253.4) ([Fig. 1]). The single nucleotide substitution of C > T results in an amino acid substitution of glutamine for arginine at codon 1118 (c.3353G > A; p.Arg1118Gln) ([Fig. 2]). The observed KDR gene variant lies in the protein tyrosine and serine/threonine kinase domain of the KDR protein. Though there are no proven clinical studies yet, and in silico analysis of the KDR gene variant p. Arg1118Gln was found to be probably damaging by PolyPhen-2 (HumVar and HumDiv) and deleterious by SIFT.
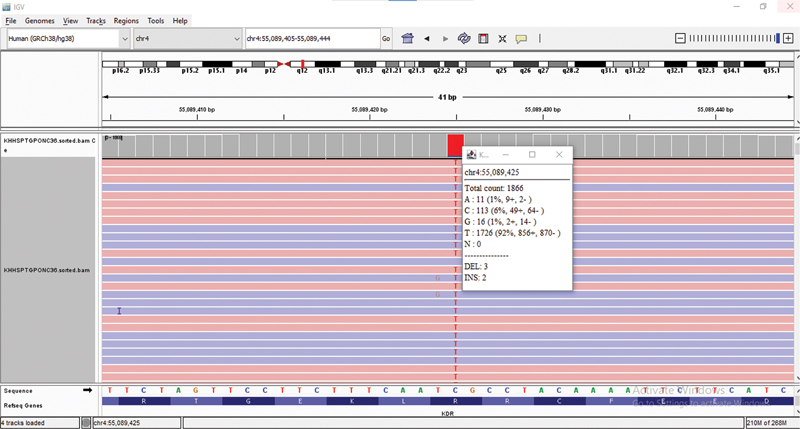
| Fig 1 :KDR Gene Variant in IGV.
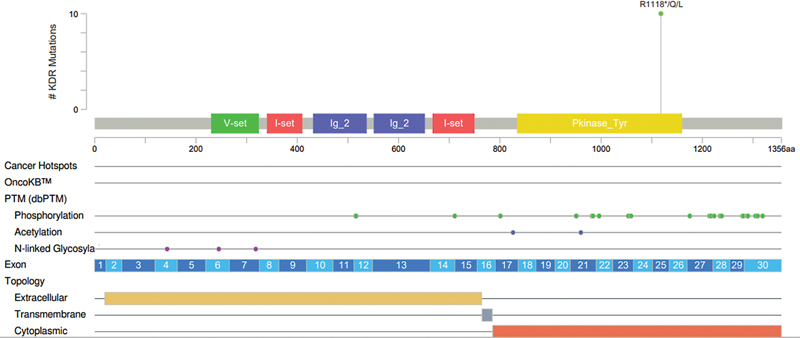
| Figure 2:Location of KDR Gene Variant (p.Arg1118Gln) (1).
Discussion
The KDR gene encodes a kinase insert domain receptor, also known as VEGFR2,[3] which is a type III receptor tyrosine kinase of the VEGF gene involved in angiogenesis.[4] Angiogenesis plays an essential role in the transition from early stages of cancer to metastasis or stage IV. Strategies or medications that block VEGF-KDR signaling successfully inhibit experimental tumor growth, as this is the foremost signaling step required for the proliferating tumor endothelium.[5] It is well known that many FDA-approved drugs targeting the KDR gene (including apatinib, cabozantinib, pazopanib, and sorafenib) have been applied to treat renal, gastric, colorectal, and other cancers.[6] [7] [8] [9] A clinical trial in a Chinese cohort is going on to treat recurrent glioblastoma with apatinib.[10]
Discussion
The KDR gene encodes a kinase insert domain receptor, also known as VEGFR2,[3] which is a type III receptor tyrosine kinase of the VEGF gene involved in angiogenesis.[4] Angiogenesis plays an essential role in the transition from early stages of cancer to metastasis or stage IV. Strategies or medications that block VEGF-KDR signaling successfully inhibit experimental tumor growth, as this is the foremost signaling step required for the proliferating tumor endothelium.[5] It is well known that many FDA-approved drugs targeting the KDR gene (including apatinib, cabozantinib, pazopanib, and sorafenib) have been applied to treat renal, gastric, colorectal, and other cancers.[6] [7] [8] [9] A clinical trial in a Chinese cohort is going on to treat recurrent glioblastoma with apatinib.[10]
Association of KDR gene Mutations in Glioma
Mutations in the KDR gene have been reported in different cancer types, predominantly in melanoma and non-melanomatic skin cancers ([Supplementary Fig. S1], available online only). The p.Arg1118Gln variant in heterozygous conditions has previously been reported in glioblastoma, rectal adenocarcinoma, uterine endometrioid cancer, and colon adenocarcinoma (Resource: cBioportal).[11] [12] Two different mutations affecting the codon 1118 (p.Arg1118Ter and p.Arg1118Leu) have previously been reported in patients with glioblastoma multiforme, sarcoma, uterine endometrioid carcinoma, lung, and colon adenocarcinoma (The AACR Project GENIE Consortium, 2017).[13] KDR p.Arg1118Ter variant is present in 0.02%-of AACR GENIE cases and comprised colon adenocarcinoma, endometrial endometrioid adenocarcinoma, lung adenocarcinoma, and sarcoma and astrocytoma.[14] [15] [16]
The limitation of this case report is that the targeted therapy associated with the KDR gene in gliomas is under clinical trials only. Though the detected variant was with a very high variant allele fraction, Sanger validation in paired tumor-normal specimens may strengthen the authenticity of zygosity. Hence, the present information helps facilitate further exploration of functional analyses of KDR mutations in gliomas.
Conclusion
This is the first report of the KDR gene variant in a homozygous state reported in a patient diagnosed with glioma. Screening mutations in protein tyrosine and serine/threonine kinase domain of the KDR will provide critical insights into the development and progression of glioma in the pediatric and adult populations. Screening for germline mutations in pediatric cases, it is strongly recommended to conduct additional testing with paired tumour-normal specimens. Because GBM, the most aggressive type of glioma, is a vascular tumor, the KDR gene associated with VEGF is probably implicated in tumor growth. Therefore, in young GBM patients with germline KDR mutations, it might be prudent to give a trial of anti-vascular drugs along with the conventional treatment regimen.
Conflicts of Interest
None declared.
Authors' Contributions
All authors have contributed and approved the manuscript.
Patient consent
The authors certify that they have obtained all appropriate patient consent forms
Statement of Ethics
This retrospective review of patient data did not require ethical approval under local guidelines. Written informed consent was obtained from the patient to publish this case report.
Supplementary Material
References
- Ostrom QT, Gittleman H, Stetson L, Virk SM, Barnholtz-Sloan JS. Epidemiology of gliomas. Cancer Treat Res 2015; 163: 1-14
- Trivedi T, Panchal K, Bhalala N, Trivedi P. Prognostic significance of STAT3 gene expression in patients with glioblastoma tumors: a study from Western India. J Egypt Natl Canc Inst 2022; 34 (01) 30
- Zehir A, Benayed R, Shah RH. et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med 2017; 23 (06) 703-713
- Biterge-Sut B. A comprehensive analysis of the angiogenesis-related genes in glioblastoma multiforme vs. brain lower grade glioma. Arq Neuropsiquiatr 2020; 78 (01) 34-38
- Banerjee K, Núñez FJ, Haase S. et al. Current Approaches for Glioma Gene Therapy and Virotherapy. Front Mol Neurosci 2021; 14: 621831
- Geng R, Song L, Li J, Zhao L. The safety of apatinib for the treatment of gastric cancer. Expert Opin Drug Saf 2018; 17 (11) 1145-1150
- Cochin V, Gross-Goupil M, Ravaud A, Godbert Y, Le Moulec S. [Cabozantinib: Mechanism of action, efficacy and indications]. . [Cabozantinib: Mechanism of action, efficacy and indications] Bull Cancer 2017; 104 (05) 393-401
- Noguerido A, Mulet-Margalef N, Matos I. et al. The safety of ramucirumab for the treatment of colorectal cancer. Expert Opin Drug Saf 2018; 17 (09) 945-951
- Choi YJ, Kim HS, Park SH. et al. Phase II study of Dovitinib in patients with castration-resistant prostate cancer (KCSG-GU11-05). Cancer Res Treat 2018; 50 (04) 1252-1259
- Glioblastoma Response Prediction to Apatinib. Accessed Jan 30, 2023, at: https://clinicaltrials.gov/ct2/show/NCT04814329?term=Glioblastoma&cond=KDR+gene&draw=2&rank=1
- Cerami E, Gao J, Dogrusoz U. et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. [published correction appears in Cancer Discov 2012;2:960] Cancer Discov 2012; 2 (05) 401-404
- Gao J, Aksoy BA, Dogrusoz U. et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 2013; 6 (269) pl1
- AACR Project GENIE Consortium. AACR Project GENIE: powering precision medicine through an international consortium. Cancer Discov 2017; 7 (08) 818-831
- Killela PJ, Pirozzi CJ, Reitman ZJ. et al. The genetic landscape of anaplastic astrocytoma. Oncotarget 2014; 5 (06) 1452-1457
- Sun S, Li X, Qu B, Xie K, Li J, Miao J. Association of the VEGFR2 single nucleotide polymorphism rs2305948 with glioma risk. Medicine (Baltimore) 2022; 101 (01) e28454
- Jonsson P, Lin AL, Young RJ. et al. Genomic correlates of disease progression and treatment response in prospectively characterized gliomas. Clin Cancer Res 2019; 25 (18) 5537-5547
Address for correspondence
Publication History
Article published online:
12 May 2023
© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Fig 1 :KDR Gene Variant in IGV.

| Figure 2:Location of KDR Gene Variant (p.Arg1118Gln) (1).
References
- Ostrom QT, Gittleman H, Stetson L, Virk SM, Barnholtz-Sloan JS. Epidemiology of gliomas. Cancer Treat Res 2015; 163: 1-14
- Trivedi T, Panchal K, Bhalala N, Trivedi P. Prognostic significance of STAT3 gene expression in patients with glioblastoma tumors: a study from Western India. J Egypt Natl Canc Inst 2022; 34 (01) 30
- Zehir A, Benayed R, Shah RH. et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med 2017; 23 (06) 703-713
- Biterge-Sut B. A comprehensive analysis of the angiogenesis-related genes in glioblastoma multiforme vs. brain lower grade glioma. Arq Neuropsiquiatr 2020; 78 (01) 34-38
- Banerjee K, Núñez FJ, Haase S. et al. Current Approaches for Glioma Gene Therapy and Virotherapy. Front Mol Neurosci 2021; 14: 621831
- Geng R, Song L, Li J, Zhao L. The safety of apatinib for the treatment of gastric cancer. Expert Opin Drug Saf 2018; 17 (11) 1145-1150
- Cochin V, Gross-Goupil M, Ravaud A, Godbert Y, Le Moulec S. [Cabozantinib: Mechanism of action, efficacy and indications]. . [Cabozantinib: Mechanism of action, efficacy and indications] Bull Cancer 2017; 104 (05) 393-401
- Noguerido A, Mulet-Margalef N, Matos I. et al. The safety of ramucirumab for the treatment of colorectal cancer. Expert Opin Drug Saf 2018; 17 (09) 945-951
- Choi YJ, Kim HS, Park SH. et al. Phase II study of Dovitinib in patients with castration-resistant prostate cancer (KCSG-GU11-05). Cancer Res Treat 2018; 50 (04) 1252-1259
- Glioblastoma Response Prediction to Apatinib. Accessed Jan 30, 2023, at: https://clinicaltrials.gov/ct2/show/NCT04814329?term=Glioblastoma&cond=KDR+gene&draw=2&rank=1
- Cerami E, Gao J, Dogrusoz U. et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. [published correction appears in Cancer Discov 2012;2:960] Cancer Discov 2012; 2 (05) 401-404
- Gao J, Aksoy BA, Dogrusoz U. et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 2013; 6 (269) pl1
- AACR Project GENIE Consortium. AACR Project GENIE: powering precision medicine through an international consortium. Cancer Discov 2017; 7 (08) 818-831
- Killela PJ, Pirozzi CJ, Reitman ZJ. et al. The genetic landscape of anaplastic astrocytoma. Oncotarget 2014; 5 (06) 1452-1457
- Sun S, Li X, Qu B, Xie K, Li J, Miao J. Association of the VEGFR2 single nucleotide polymorphism rs2305948 with glioma risk. Medicine (Baltimore) 2022; 101 (01) e28454
- Jonsson P, Lin AL, Young RJ. et al. Genomic correlates of disease progression and treatment response in prospectively characterized gliomas. Clin Cancer Res 2019; 25 (18) 5537-5547


 PDF
PDF  Views
Views  Share
Share

