Daratumumab
CC BY-NC-ND 4.0 ? Indian J Med Paediatr Oncol 2020; 41(01): 57-60
DOI: DOI: 10.4103/ijmpo.ijmpo_263_19
Abstract
Multiple myeloma is a proliferative disorder of plasma cells in the bone marrow with excessive monoclonal protein production. Despite the evolution of multiple drugs and management strategies including maintenance and autologous stem cell transplantation, the long-term results still remain undesirable. Those patients with double refractoriness to immunomodulatory drugs and proteosome inhibitors have dismal prognosis and rarely get back into durable remission. The identification of CD38 as a therapeutic target for multiple myeloma resulted in the clinical development of anti-CD38 antibodies. Daratumumab is an IgG1 human monoclonal antibody that binds to the CD38 protein. The implementation of daratumumab in clinical practice is widely considered as a significant milestone in the management of multiple myeloma. The salient pharmacological aspects and clinical evolution of the drug are briefly discussed in this review.

Publication History
Received: 23 December 2019
Accepted: 10 February 2020
Publication Date:
23 May 2021 (online)
? 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Multiple myeloma is a proliferative disorder of plasma cells in the bone marrow with excessive monoclonal protein production. Despite the evolution of multiple drugs and management strategies including maintenance and autologous stem cell transplantation, the long-term results still remain undesirable. Those patients with double refractoriness to immunomodulatory drugs and proteosome inhibitors have dismal prognosis and rarely get back into durable remission. The identification of CD38 as a therapeutic target for multiple myeloma resulted in the clinical development of anti-CD38 antibodies. Daratumumab is an IgG1 human monoclonal antibody that binds to the CD38 protein. The implementation of daratumumab in clinical practice is widely considered as a significant milestone in the management of multiple myeloma. The salient pharmacological aspects and clinical evolution of the drug are briefly discussed in this review.
Keywords
CD38 - daratumumab - multiple myelomaIntroduction
Multiple myeloma is an incurable plasma cell dyscrasia. In India, the age-adjusted incidence rates of multiple myeloma are 1.13 and 0.81/100,000 men and women, respectively.[1] Despite the advances in treatment with the use of immunomodulatory drugs (IMiDs) and proteosome inhibitors (PIs), patients with multiple myeloma typically have multiple relapses. Hence, there is a continuous requirement of new drugs which can increase the depths and durations of responses and can probably attempt at reversing the incurable nature of the disease. Since the first approval of daratumumab monotherapy in relapsed refractory multiple myeloma in November 2015, it has come a long way in addressing some of these unmet needs in multiple myeloma treatment.
Mechanism of Action and Essential Pharmacology
CD38 is a transmembrane glycoprotein receptor which is highly expressed on the surface of plasma cells and is involved in multiple functions including cell adhesion, signaling, and modulation of enzymatic activities.[2] Daratumumab after binding to CD38 causes cell death through various mechanisms as illustrated in [Figure 1].[3] [4]
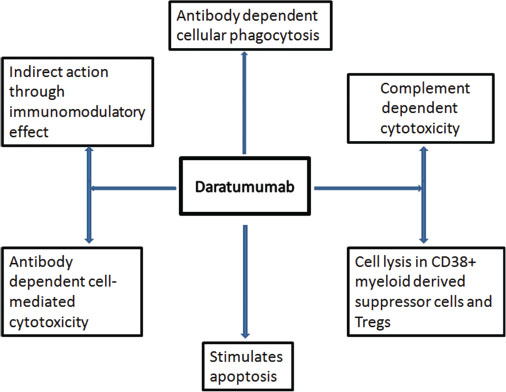
|?Fig. 1: Mechanisms of action of daratumumab
Daratumumab elimination exhibits nonlinear characteristics, suggesting the target-mediated drug deposition.[5] The half-life increases with increasing doses and multiple doses, and the clearance at a dose of 16 mg/kg was estimated at 9.0 ? 4.3 days after the first dose and at 10.6 ? 9.0 days after varying numbers of repeated doses in the GEN501 study.[6] The elimination half-life using a specific pharmacokinetic model was found to be 21 days.[7] The pharmacokinetics also varies depending on whether daratumumab is given as a single agent or in combination. The minimum effective dose to achieve the required target saturation and overall response rates is 16 mg/kg.[5] Clinically relevant drug interactions are unknown at present.[8] Daratumumab can also be safely administered regardless of the baseline renal and hepatic functions.[9] [10]
Indications
The Food and Drug Administration-approved indications are:
Relapsed refractory myeloma ? In combination with Rd (lenalidomide and dexamethasone ? Daratumumab, lenalidomide, dexamethasone [DRd] regimen) or Vd (bortezomib and dexamethasone ? Daratumumab, bortezomib, dexamethasone regimen), after at least one prior therapy or with Pd (pomalidomide and dexamethasone ? Daratumumab, pomalidomide, dexamethasone regimen) after at least two prior therapies
Relapsed refractory myeloma ? As monotherapy for the treatment of patients with multiple myeloma who have received at least three prior lines of therapy including a PI and an IMiD or who are double refractory to a PI and an IMiD
First-line therapy ? In combination with VTd (bortezomib, thalidomide, and dexamethasone ? DVTd) in newly diagnosed patients who are eligible for autologous stem cell transplant (ASCT)
First-line therapy ? In combination with Rd (as DRd) or in comibination with VMP (Daratumumab, bortezomib, melphalan, prednisone regimen - DVMP) for patients with newly diagnosed multiple myeloma who are ineligible for ASCT.
Clinical efficacy ? major trials [Table 1]
Table 1 Major trials which led to Food and Drug Administration approval of various indications
Dosage and administration
Vial strength ? 100 mg/5 ml and 400 mg/20 ml.
Premedication ? Premedicate with a corticosteroid (methylprednisolone at 100 mg IV or dexamethasone at 20 mg IV), antihistamine (diphenhydramine at 25?50 mg PO or IV), and oral acetaminophen at 650?1000 mg. These should be administered 30?60 min before the infusion
Administration ? The infusion set should be fitted with a flow regulator and an inline low protein-binding polyethersulfone filter (0.2 ?m). The infusion bag should not be shaken at any point of time
First infusion ? Administer daratumumab at 16 mg/kg (in 1000 ml), starting at 50 ml/h. It can be increased at a rate increment of 50 ml/h to a maximum of 200 ml/h
Subsequent infusions - In 500 ml, starting at 100 ml/h. It can be escalated by a rate increment of 50 m/h to a maximum of 200 m/h
Postinfusion medication ? Administer an oral corticosteroid (20-mg prednisolone) on each of the 2 days after the infusion. Prophylaxis against herpes zoster reactivation should be initiated within 1 week of the first infusion, and it has to be continued for 3 months after the last dose. Postinfusion medication is only for the 1st?cycle unless otherwise indicated
The commonly used schedules of daratumumab are given in [Table 2].
Adverse Events
Around 50% of the patients will develop infusion-related reactions (IRRs) which are mostly of Grade 1 and 2 and it rarely results in drug discontinuation.[6] Apart from IRRs, it is a relatively safer drug to administer and the major adverse events noted in the 148 patients who received daratumumab monotherapy are listed in [Table 3].[6] [12] [18] In randomized trials involving multidrug regimens, the daratumumab combinations resulted in increased cytopenias (mainly neutropenia/thrombocytopenia) in majority of the trials[12] [13] [14] [16] and increased infection rates in some trials,[16] [17] but none were considered to be clinically significant. No dose reductions are recommended for toxicities. It should be permanently discontinued after a Grade 4 infusion reaction. Dose adjustments are not required for hepatic dysfunction and for a creatine clearance of 15?89 ml/min.
Table 3Major adverse events with monotherapy
|
Adverse events |
Any grade, n (%) |
Grade 3-4, n (%) |
|---|---|---|
|
URTI ? Upper respiratory tract infection |
||
|
Hematological |
||
|
Anemia |
42 (28.4) |
26 (17.6) |
|
Thrombocytopenia |
32 (21.6) |
21 (14.2) |
|
Neutropenia |
31 (20.9) |
15 (10.1) |
|
Nonhematological |
||
|
Fatigue |
62 (41.9) |
3 (2) |
|
Nausea |
44 (29.7) |
0 |
|
Cough |
38 (25.7) |
0 |
|
URTI |
32 (21.6) |
1 (0.7) |
Special Considerations
Interference with serological testing
The binding of daratumumab to red blood cells (RBCs) which also expresses CD38 can result in false-positive indirect antiglobulin tests, and it can prevent the identification of antibodies and can cause a delay in the issue of compatible products from blood banks.[19] Daratumumab can result in positive indirect antiglobulin tests up to 6 months after exposure.[20] Dithiothreitol (DTT) is an agent which can remove and nullify the effects of daratumumab to serological testing. Hence, treating the RBCs with DTT can help in the issue of the safer blood products to daratumumab recipients. Kell antigen-negative blood products are recommended for daratumumab recipients as it can cause destruction of Kell antigens.[21] Other approaches suggested for nullifying the daratumumab effect include the addition of soluble CD38 or antidaratumumab idiotypic antibodies.[22]
Interference with determination of complete response
While doing electrophoresis and immunofixation for response assessment in IgG kappa multiple myelomas, false-positive results can occur as daratumumab is an IgG kappa antibody. This can be avoided by performing response assessment using serum-free light chain assay, daratumumab-specific immunofixation electrophoresis, or mass spectroscopy.[23] [24]
Cost in India
Approximately 1.5 lakh rupees for 800 mg.
Conclusion
Daratumumab is one of the path-breaking drugs in the field of multiple myeloma therapeutics. The activity has been demonstrated in almost all lines of myeloma therapy except maintenance. The addition of daratumumab to conventional regimens has improved the depth and sustainability of response and improved survivals in majority of the studies. The high cost is the only factor which is precluding the regular use in routine clinical practice in the Indian settings.
Conflict of Interest
There are no conflicts of interest.
References
- Bora K.?Distribution of multiple myeloma in India: Heterogeneity in incidence across age, sex and geography. Cancer Epidemiol 2019; 59: 215-20
- Overdijk MB, Verploegen S, B?gels M, van Egmond M, Lammerts van Bueren JJ, Mutis T. et al.?Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. MAbs 2015; 7: 311-21
- Sanchez L, Wang Y, Siegel DS, Wang ML.?Daratumumab: A first-in-class CD38 monoclonal antibody for the treatment of multiple myeloma. J Hematol Oncol 2016; 9: 51
- Krejcik J, Casneuf T, Nijhof IS, Verbist B, Bald J, Plesner T. et al.?Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood 2016; 128: 384-94
- Clemens PL, Yan X, Lokhorst HM, Lonial S, Losic N, Khan I. et al.?Pharmacokinetics of daratumumab following intravenous infusion in relapsed or refractory multiple myeloma after prior proteasome inhibitor and immunomodulatory drug treatment. Clin Pharmacokinet 2017; 56: 915-24
- Lokhorst HM, Plesner T, Laubach JP, Nahi H, Gimsing P, Hansson M. et al.?Targeting CD38 with daratumumab monotherapy in multiple myeloma. N Engl J Med 2015; 373: 1207-19
- Phipps C, Chen Y, Gopalakrishnan S, Tan D.?Daratumumab and its potential in the treatment of multiple myeloma: Overview of the preclinical and clinical development. Ther Adv Hematol 2015; 6: 120-7
- Khagi Y, Mark TM.?Potential role of daratumumab in the treatment of multiple myeloma. Onco Targets Ther 2014; 7: 1095-100
- Cavo M, Dimopoulos MA, San-Miguel J, Jakubowiak AJ, Suzuki K, Yoon SS. et al.?Impact of baseline renal function on efficacy and safety of daratumumab plus bortezomib-melphalan-prednisone (VMP) in patients (Pts) with newly diagnosed multiple myeloma (NDMM) ineligible for transplantation (ALCYONE). J Clin Oncol 2018; 36 (Suppl. 15) e20024
- Raedler LA.?Darzalex (daratumumab):First anti-CD38 monoclonal antibody approved for patients with relapsed multiple myeloma. Am Health Drug Benefits 2016; 9: 70-3
- Lonial S, Weiss BM, Usmani SZ, Singhal S, Chari A, Bahlis NJ. et al.?Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): An open-label, randomised, phase 2 trial. Lancet 2016; 387: 1551-60
- Dimopoulos MA, Oriol A, Nahi H, San-Miguel J, Bahlis NJ, Usmani SZ. et al.?Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med 2016; 375: 1319-31
- Palumbo A, Chanan-Khan A, Weisel K, Nooka AK, Masszi T, Beksac M. et al.?Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med 2016; 375: 754-66
- Chari A, Suvannasankha A, Fay JW, Arnulf B, Kaufman JL, Ifthikharuddin JJ. et al.?Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood 2017; 130: 974-81
- Mateos MV, Dimopoulos MA, Cavo M, Suzuki K, Jakubowiak A, Knop S. et al.?Daratumumab plus bortezomib, melphalan, and prednisone for untreated myeloma. N Engl J Med 2018; 378: 518-28
- Facon T, Kumar S, Plesner T, Orlowski RZ, Moreau P, Bahlis N. et al.?Daratumumab plus lenalidomide and dexamethasone for untreated myeloma. N Engl J Med 2019; 380: 2104-15
- Moreau P, Attal M, Hulin C, Arnulf B, Belhadj K, Benboubker L. et al.?Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): A randomised, open-label, phase 3 study. Lancet 2019; 394: 29-38
- Usmani SZ, Weiss BM, Plesner T, Bahlis NJ, Belch A, Lonial S. et al.?Clinical efficacy of daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma. Blood 2016; 128: 37-44
- Sullivan HC, Gerner-Smidt C, Nooka AK, Arthur CM, Thompson L, Mener A. et al.?Daratumumab (anti-CD38) induces loss of CD38 on red blood cells. Blood 2017; 129: 3033-7
- Oostendorp M, Lammerts van Bueren JJ, Doshi P, Khan I, Ahmadi T, Parren PW. et al.?When blood transfusion medicine becomes complicated due to interference by monoclonal antibody therapy. Transfusion 2015; 55: 1555-62
- Chapuy CI, Nicholson RT, Aguad MD, Chapuy B, Laubach JP, Richardson PG. et al.?Resolving the daratumumab interference with blood compatibility testing. Transfusion 2015; 55: 1545-54
- Nooka AK, Kaufman JL, Hofmeister CC, Joseph NS, Heffner TL, Gupta VA. et al.?Daratumumab in multiple myeloma. Cancer 2019; 125: 2364-82
- Rosenberg AS, Bainbridge S, Pahwa R, Jialal I.?Investigation into the interference of the monoclonal antibody daratumumab on the free light chain assay. Clin Biochem 2016; 49: 1202-4
- McCudden C, Axel AE, Slaets D, Dejoie T, Clemens PL, Frans S. et al.?Monitoring multiple myeloma patients treated with daratumumab: Teasing out monoclonal antibody interference. Clin Chem Lab Med 2016; 54: 1095-104
Address for correspondence
Publication History
Received: 23 December 2019
Accepted: 10 February 2020
Publication Date:
23 May 2021 (online)
? 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

|?Fig. 1: Mechanisms of action of daratumumab
References
- Bora K.?Distribution of multiple myeloma in India: Heterogeneity in incidence across age, sex and geography. Cancer Epidemiol 2019; 59: 215-20
- Overdijk MB, Verploegen S, B?gels M, van Egmond M, Lammerts van Bueren JJ, Mutis T. et al.?Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. MAbs 2015; 7: 311-21
- Sanchez L, Wang Y, Siegel DS, Wang ML.?Daratumumab: A first-in-class CD38 monoclonal antibody for the treatment of multiple myeloma. J Hematol Oncol 2016; 9: 51
- Krejcik J, Casneuf T, Nijhof IS, Verbist B, Bald J, Plesner T. et al.?Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood 2016; 128: 384-94
- Clemens PL, Yan X, Lokhorst HM, Lonial S, Losic N, Khan I. et al.?Pharmacokinetics of daratumumab following intravenous infusion in relapsed or refractory multiple myeloma after prior proteasome inhibitor and immunomodulatory drug treatment. Clin Pharmacokinet 2017; 56: 915-24
- Lokhorst HM, Plesner T, Laubach JP, Nahi H, Gimsing P, Hansson M. et al.?Targeting CD38 with daratumumab monotherapy in multiple myeloma. N Engl J Med 2015; 373: 1207-19
- Phipps C, Chen Y, Gopalakrishnan S, Tan D.?Daratumumab and its potential in the treatment of multiple myeloma: Overview of the preclinical and clinical development. Ther Adv Hematol 2015; 6: 120-7
- Khagi Y, Mark TM.?Potential role of daratumumab in the treatment of multiple myeloma. Onco Targets Ther 2014; 7: 1095-100
- Cavo M, Dimopoulos MA, San-Miguel J, Jakubowiak AJ, Suzuki K, Yoon SS. et al.?Impact of baseline renal function on efficacy and safety of daratumumab plus bortezomib-melphalan-prednisone (VMP) in patients (Pts) with newly diagnosed multiple myeloma (NDMM) ineligible for transplantation (ALCYONE). J Clin Oncol 2018; 36 (Suppl. 15) e20024
- Raedler LA.?Darzalex (daratumumab):First anti-CD38 monoclonal antibody approved for patients with relapsed multiple myeloma. Am Health Drug Benefits 2016; 9: 70-3
- Lonial S, Weiss BM, Usmani SZ, Singhal S, Chari A, Bahlis NJ. et al.?Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): An open-label, randomised, phase 2 trial. Lancet 2016; 387: 1551-60
- Dimopoulos MA, Oriol A, Nahi H, San-Miguel J, Bahlis NJ, Usmani SZ. et al.?Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med 2016; 375: 1319-31
- Palumbo A, Chanan-Khan A, Weisel K, Nooka AK, Masszi T, Beksac M. et al.?Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med 2016; 375: 754-66
- Chari A, Suvannasankha A, Fay JW, Arnulf B, Kaufman JL, Ifthikharuddin JJ. et al.?Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood 2017; 130: 974-81
- Mateos MV, Dimopoulos MA, Cavo M, Suzuki K, Jakubowiak A, Knop S. et al.?Daratumumab plus bortezomib, melphalan, and prednisone for untreated myeloma. N Engl J Med 2018; 378: 518-28
- Facon T, Kumar S, Plesner T, Orlowski RZ, Moreau P, Bahlis N. et al.?Daratumumab plus lenalidomide and dexamethasone for untreated myeloma. N Engl J Med 2019; 380: 2104-15
- Moreau P, Attal M, Hulin C, Arnulf B, Belhadj K, Benboubker L. et al.?Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): A randomised, open-label, phase 3 study. Lancet 2019; 394: 29-38
- Usmani SZ, Weiss BM, Plesner T, Bahlis NJ, Belch A, Lonial S. et al.?Clinical efficacy of daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma. Blood 2016; 128: 37-44
- Sullivan HC, Gerner-Smidt C, Nooka AK, Arthur CM, Thompson L, Mener A. et al.?Daratumumab (anti-CD38) induces loss of CD38 on red blood cells. Blood 2017; 129: 3033-7
- Oostendorp M, Lammerts van Bueren JJ, Doshi P, Khan I, Ahmadi T, Parren PW. et al.?When blood transfusion medicine becomes complicated due to interference by monoclonal antibody therapy. Transfusion 2015; 55: 1555-62
- Chapuy CI, Nicholson RT, Aguad MD, Chapuy B, Laubach JP, Richardson PG. et al.?Resolving the daratumumab interference with blood compatibility testing. Transfusion 2015; 55: 1545-54
- Nooka AK, Kaufman JL, Hofmeister CC, Joseph NS, Heffner TL, Gupta VA. et al.?Daratumumab in multiple myeloma. Cancer 2019; 125: 2364-82
- Rosenberg AS, Bainbridge S, Pahwa R, Jialal I.?Investigation into the interference of the monoclonal antibody daratumumab on the free light chain assay. Clin Biochem 2016; 49: 1202-4
- ?McCudden C, Axel AE, Slaets D, Dejoie T, Clemens PL, Frans S. et al.?Monitoring multiple myeloma patients treated with daratumumab: Teasing out monoclonal antibody interference. Clin Chem Lab Med 2016; 54: 1095-104


 PDF
PDF  Views
Views  Share
Share

