Diarrhea in neutropenic children with cancer: An Egyptian center experience, with emphasis on neutropenic enterocolitis
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2012; 33(02): 95-101
DOI: DOI: 10.4103/0971-5851.99742
Abstract
Background: Diarrhea is a frequent complication in children with cancer who received intensive chemotheraputic regimens. It may be caused by several factors, neutropenic enterocolitis (NE) being the most serious. Aim: To study diarrhea in neutropenic cancer patients in the pediatric age group, with its underlying etiologies and risk factors, especially the bacterial causes, with special concern on NE. Materials and Methods: This study was carried out at the Pediatric Hematology and Oncology Units, Zagazig University Hospitals, Egypt, from January 2009 to September 2010. All children with malignant diseases who are ≤12 years of age were included. Patients who were neutropenic ( < 500/ mm 3 ) on admission or who became neutropenic during their stay in the hospital were monitored regularly (daily) for diarrhea. Neutropenic cancer patients with diarrhea were grouped into two groups: Group 1, with NE, and group 2, with neutropenic diarrhea rather than NE. On the first day of diarrhea, patients were subjected to complete blood count, blood cultures, stool microscopy and culture. Abdominal ultrasonography was carried out within 3 days of diarrhea. Results: A total of 200 children ≤12 years old, suffering from different malignancies, with a total of 180 neutropenic episodes were followed. Diarrhea was observed in 100 episodes (55.5%). NE constituted 16% of these diarrheal episodes. All patients with NE had significantly more severe neutropenia, and this was of longer duration than the other group. All patients with NE were febrile, with 100% positive blood culture. Stool analysis diagnosed giardiasis in 4.8% of the non-NE patients and in none of the NE patients, while stool culture was positive in 75% of the NE patients compared with 40.5% of the other group. Conclusions: Diarrhea is a common complication in neutropenic cancer children. Gram negative bacteria and Candida are the most incriminated pathogens. Duration and severity of neutropenia carry a great risk for the development of NE.
Publication History
Article published online:
13 April 2022
© 2012. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:
Diarrhea is a frequent complication in children with cancer who received intensive chemotheraputic regimens. It may be caused by several factors, neutropenic enterocolitis (NE) being the most serious.
Aim:
To study diarrhea in neutropenic cancer patients in the pediatric age group, with its underlying etiologies and risk factors, especially the bacterial causes, with special concern on NE.
Materials and Methods:
This study was carried out at the Pediatric Hematology and Oncology Units, Zagazig University Hospitals, Egypt, from January 2009 to September 2010. All children with malignant diseases who are ≤12 years of age were included. Patients who were neutropenic ( < 500/ mm3) on admission or who became neutropenic during their stay in the hospital were monitored regularly (daily) for diarrhea. Neutropenic cancer patients with diarrhea were grouped into two groups: Group 1, with NE, and group 2, with neutropenic diarrhea rather than NE. On the first day of diarrhea, patients were subjected to complete blood count, blood cultures, stool microscopy and culture. Abdominal ultrasonography was carried out within 3 days of diarrhea.
Results:
A total of 200 children ≤12 years old, suffering from different malignancies, with a total of 180 neutropenic episodes were followed. Diarrhea was observed in 100 episodes (55.5%). NE constituted 16% of these diarrheal episodes. All patients with NE had significantly more severe neutropenia, and this was of longer duration than the other group. All patients with NE were febrile, with 100% positive blood culture. Stool analysis diagnosed giardiasis in 4.8% of the non-NE patients and in none of the NE patients, while stool culture was positive in 75% of the NE patients compared with 40.5% of the other group.
Conclusions:
Diarrhea is a common complication in neutropenic cancer children. Gram negative bacteria and Candida are the most incriminated pathogens. Duration and severity of neutropenia carry a great risk for the development of NE.
INTRODUCTION
Diarrhea is a frequent complication in children with cancer.[1] Possible etiologies include radiotherapy, chemotherapy, graft versus host disease and infections.[2]
Diarrhea can be debilitating and, in some cases, life-threatening, causing volume depletion, renal failure and electrolyte disorders.[3]
Neutropenic enterocolitis (NE) is a specific disease entity, usually manifesting itself with diarrhea.[4] It is a severe complication of chemotherapeutic regimens.[5] The acute inflammatory disease may involve cecum, colon and the terminal part of the ileum.[6] The mechanism by which NE occurs is mutifactorial and thought to result from a combination of multiple factors, including neutropenia, chemotherapy- or radiotherapy-induced destruction of normal mucosa, intramural hemorrhage caused by severe thrombocytopenia and change in normal gastrointestinal flora caused by antibiotics, antifungal agents and colonization by hospital flora.[7] Prolonged neutropenia appears to be the pre-requisite factor in conjunction with injury to the bowel mucosa.[8]
The diagnosis of NE may be delayed because the presenting clinical features (fever, abdominal pain and diarrhea) are not specific and may suggest other abdominal diseases.[9,10] To support the clinical diagnosis of NE, imaging techniques have therefore been used, including abdominal ultrasonography[11,12] and computed tomography.[13–15]
Current data on diarrhea in neutropenic cancer children are limited.[5] We conducted a prospective study over a period of 21 months to determine the incidence, risk factors and causes of diarrhea in neutropenic children with cancer.
MATERIALS AND METHODS
The current study was conducted at the Pediatric Hematology and Oncology Units, Zagazig University Hospitals, Egypt, from January 2009 to September 2010 on children with malignant diseases ≤12 years of age regardless of their underlying malignancy and phase of treatment. Patients who were neutropenic (≤500/ mm3) on admission or who became neutropenic during their hospital stay were monitored regularly (daily) until discharge or recovery from neutropenia, whichever occurred earlier, to monitor the presence of diarrhea. Those who developed diarrhea were grouped into two groups: Group 1, with NE, and group 2, with neutropenic diarrhea rather than NE. The study was approved by the research and ethical committee - Zagazig university.
All the parents of eligible children were consented at the beginning of the study for inclusion of their children in the study.
Data regarding demographic characteristics (age, gender, underlying disease and the status of the disease), duration and severity of neutropenia and risk factors for diarrhea were recorded. All patients were subjected to complete history taking and thorough clinical examination. Vital signs and physical examination findings were monitored regularly (daily) in patients who developed diarrhea till recovery. Routine investigations included complete blood count (CBC), creatinine, urea, alanine transaminase, aspartate transaminase, sodium (Na), potassium (K), C-reactive protein (CRP) and arterial blood gases.
On the first day of diarrhea, CBC, blood cultures, stool microscopy for red and white blood cells, parasitic cysts and ova examination and stool culture for specific pathogens were collected. All microbiological tests were repeated on the following day.
For isolation of Salmonellae and Shigellae, enrichment using tetrathionate broth for 18-24 h was performed, followed by plating on Salmonella-Shigella agar and incubation at 37°C for 18-24 h. For isolation of Campylobacter,[16] Skirrow's Campylobacter medium with vancomycin, polymyxin B and trimethoprim and aerotolerance growth supplements (Oxoid) were used and incubated for 48 h at 42-43°C in a candle jar. For isolation of Enterohemorrhagic E. coli (E. coli O157), specimens were plated on Sorbitol MacConkey's agar.[17] Stool samples were also inoculated on blood agar (5%) and MacConkeys agar (Oxoid) and incubated at 37°C for 18-24 h. Colonies were identified by Gram stain, conventional biochemical tests and Api20E (BioMérieux, Marcy, L'Etoile, France). Quantitative cultures were carried out for Candida spp. 0.1 ml of the 1/10 diluted stool sample was inoculated into sabouraud dextrose agar and incubated at room temperature for 24-48 h ≥105 colonies considered significant).[18] Blood cultures were carried out using Oxoid and then subcultured on blood and MacConkeys agar. Gram +ve cocci were identified as enterococci by their ability to grow on MacConkey broth containing 6.5% NaCl and esculin hydrolysis.[19]
Abdominal ultrasonography was carried out very early, within 3 days of the onset of diarrhea, for assessment of bowel wall thickness (thickness ≥3 mm was considered abnormal), pneumatosis intestinalis, irregularity of bowel loop, mucosal enhancement and dilatation of bowel loop, aiming to differentiate NE from neutropenic diarrhea due to other causes.
RESULTS
A total of 200 children ≤12 years old, range 9 months to 12 years, suffering from different malignancies were included in the current study. Forty-six percent of them were males and 54% were females. A total of 180 neutropenic episodes in these patients were followed. The underlying disease was a hematological malignancy in most (92%) of the cases [Table 1]. Diarrhea was observed in 100 episodes (55.5%). NE constituted 16% of these diarrheal episodes. Cytarabine (48%) was the most common cytotoxic chemotherapeutic agent used in our patients. Methotexate and mitoxantrone were associated significantly with the development of NE [Table 2]. All the patients who developed diarrhea received antimicrobial therapy before the onset of diarrhea. Co-trimoxazole, ceftazidime and metronidazile were the most commonly used while carbapenems were most commonly associated with NE (P=0.02 for imipenem and 0.001 for meropenem).
Table 1
Demographic characteristics of the patients studied
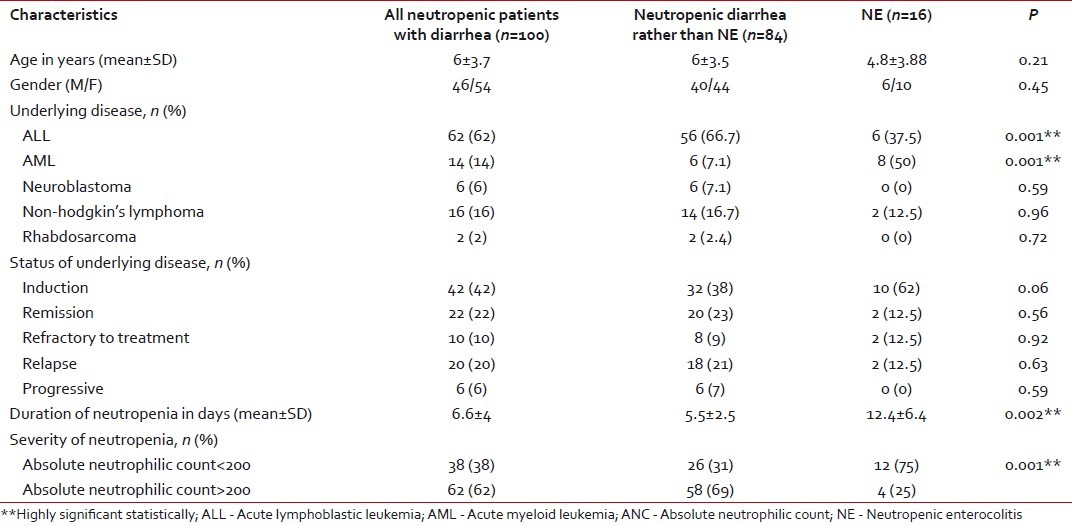
Table 2
Chemotheraputic agents used in the treatment of the study population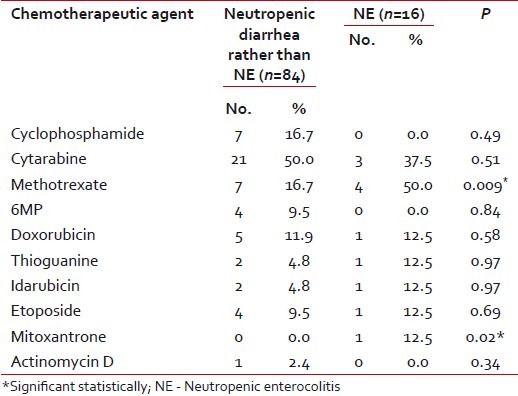
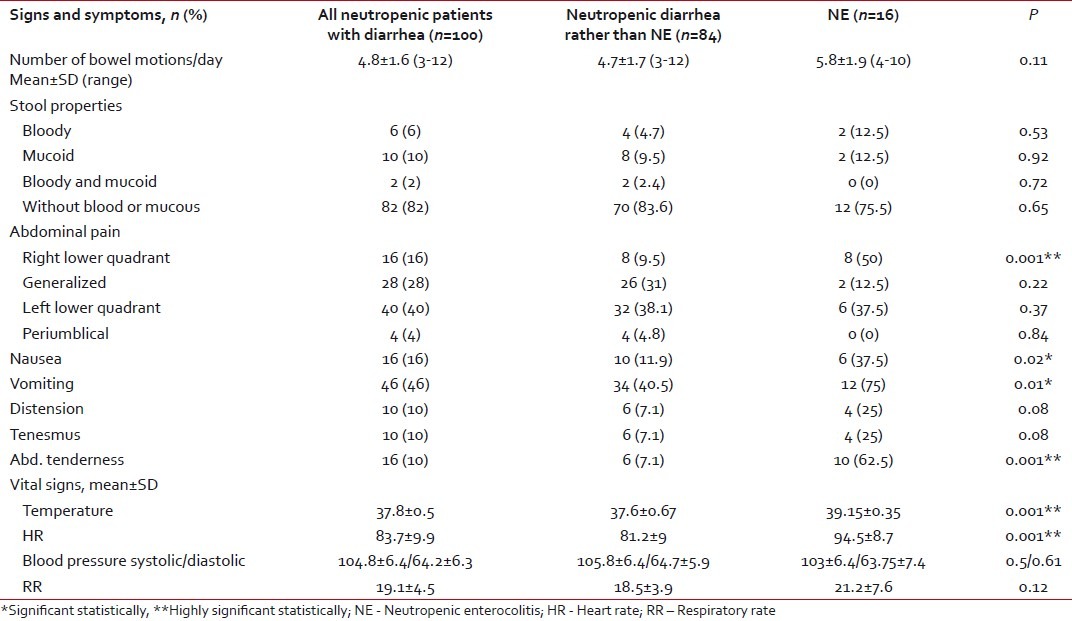
Acute leukemia was the most common malignancy in both groups. Acute lymphoblastic leukemia was the most prevalent in diarrhea other than NE (66.7%), while acute myeloid leukemia dominated in patients with NE (50%). Most of the diarrheal episodes occurred during the induction phase of chemotherapy (42%). Neutropenia was significantly longer in duration and more severe in patients with NE compared with the other group (P < 0.001) [Table 1]. There was no mortality recorded in either group. Abdominal pain was the most frequent symptom in all patients (88%). Right lower quadrant abdominal pain and abdominal tenderness with increased body temperature and heart rate were the significant clinical findings in patients with NE when compared with the other group. The mean number of bowel motions in patients was 4.8 per day, with no significant difference between the two groups. In 82% of the patients, the stool had no mucous or blood [Table 3].
Table 3
Clinical characteristics of the studied patients with diarrhea
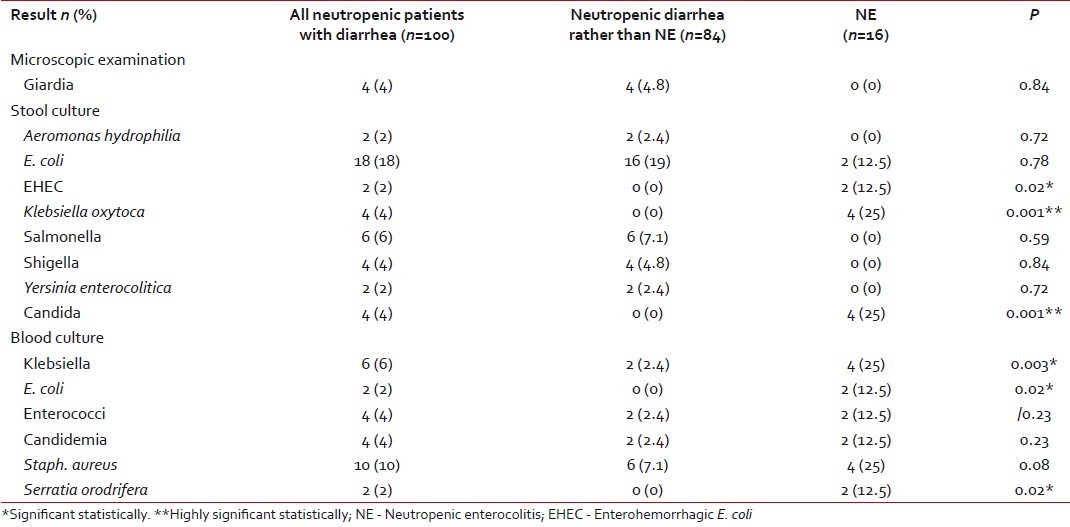
High fever and positive CRP were indefinite findings in all patients with NE compared with 38% with fever and 45.2% with positive CRP in the other group, with high statistical significance (P=0.001) [Table 2]. This might indicate that diarrhea in patients with NE was caused by infectious agents. This was supported by the positive blood culture in all NE patients compared with only 37.5% in the other group (P=0.001). Stool culture was positive in 75% of the NE patients compared with 40.5% in the other group, with no statistical difference. The most common isolated organisms in stool were Klebsiella oxytoca and Candida in patients with NE, while it was E. coli in the second group. Giardiasis was detected in the stool samples of four patients from the second group. All these data are presented in Tables Tables44 and and55.
Table 4
Laboratory findings in the studied population
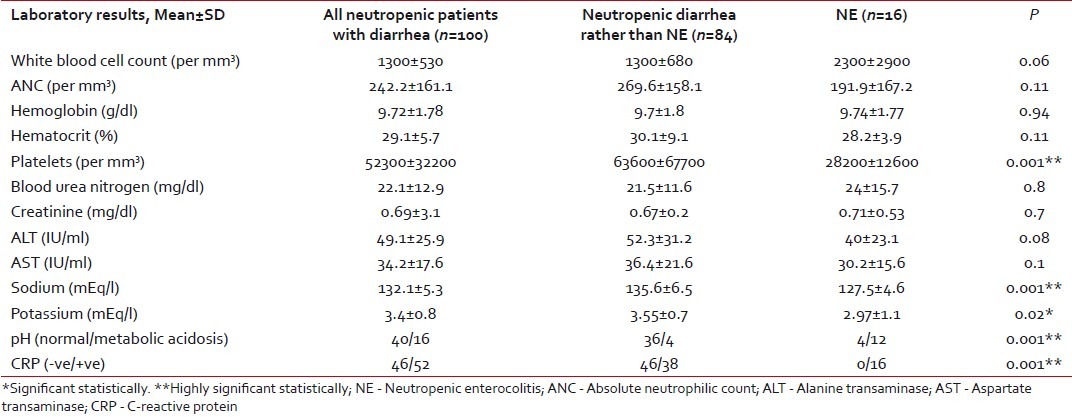
Table 5
Microbial evaluation of the stool and blood samples in the studied patients
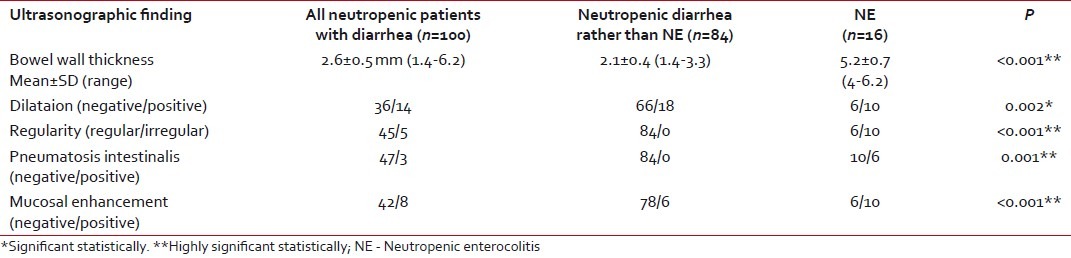
Ultrasonographic evaluation using a high-resolution probe was carried out in all the studied patients [Table 6]. Bowel wall thickness was increased significantly in patients with NE (mean: 5.2±0.7 mm) than in the other group (mean: 2.1±0.4 mm), with P < 0.001. Pneumatosis intestinalis and irregular bowel wall were solely found in patients with NE, P < 0.001. There was a highly significant mucosal enhancement (P < 0.001) and significant bowel wall dilatation in patients with NE when compared with the second group (P=0.002).
Table 6
Abdominal ultrasonographic finding in the studied patients
DISCUSSION
Diarrhea is a frequent complication of cytotoxic chemotherapy; its true incidence, risk factors and clinical course have rarely been investigated prospectively. To the best of our knowledge, this is the largest prospective study in children to date, which evaluates diarrhea in neutropenic children with cancer. Most of the data in the literature rely on case reports, case series and retrospective studies.[20] The study carried by McCarville et al.[21] is the largest retrospective study to date on typhlitis in childhood cancer, which discussed 83 children with typhlitis.
In the current study, diarrhea was observed in 55.5% (100 episodes) of all episodes of neutropenia (180 episodes) experienced by the study group.
In our study, we used specific quantifiable bowel wall measurements of ≥3 mm obtained by ultrasonography (US) imaging in addition to the constellation of neutropenia and diarrhea and/or abdominal pain and/or fever to label the patients as having NE.
The reported incidences of NE in literature in adults vary considerably from 0.8% to 26%,[22,23] while studies in pediatric population demonstrated incidence rates of 0.35-6.1%.[24,25] These results, although variable in different patient populations, underscore the importance of NE as a clinical problem.[7]
These variations can be explained on the basis of the patient population studied and that in most of these studies, the focus was on typhlitis, which may be regarded as a localized form of NE limited to caecal inflammation.[26]
We found a clear significant high incidence of NE (16%) among the children treated for cancer at our institution as compared to other studies. We attribute this increase, at least in part, to our policy of performing abdominal ultrasonography very early within 3 days from the development of diarrhea in neutropenic children without waiting for the clinical triad of diarrhea, fever and abdominal pain to be fulfilled. We found that the clinical triad is always completed during the course of the hospital stay, indicating that this policy helped to pick up early cases before the development of the full blown picture of NE, which is later reflected on the favorable outcome with resolution of NE without any surgical morbidity and without any reported mortality. The difference in mortality rate as compared with other studies in literature might also be attributed to the difference in the age group of the studied population, which was ≤12 year old in our study. The two mortalities in the McCarville's[21] study group were in 14- and 15-years old. Overall, patients >16 years old were at a significantly greater risk of NE and its associated morbidity and mortality than younger patients. Age is noteworthy a significant demographic variable associated with the development of typhlitis.[21] Older patients not only are at a greater risk of typhlitis but may also not respond as well as compared to younger patients to its management.[25]
In agreement with McCarville et al.,[21] the imaging and clinical features that we found to be significantly associated with NE were the duration and severity of neutropenia, bowel wall thickness as measured by US imaging, fever and abdominal tenderness. Jain et al.[25] and Aksoy et al.[4] also agreed with us regarding the duration of neutropenia. Aksoy et al.[4] agreed as well as regards the severity of neutropenia while Dietrich et al.[27] had significant results like ours regarding fever.
Right lower quadrant abdominal pain was another significant clinical finding in patients with NE. That was in agreement with the results of Merine et al.[28] and Alioglu et al.[29]
The development of typhlitis has historically been attributed to previous drug therapy.[21] Our findings suggest that several drugs can affect the development of typhlitis in children. Methotrexate and mitoxantrone were significantly associated with NE in our patients, while anthracyclines showed no significant statistical difference in our work. Akosy et al.[4] agreed with us regarding mitoxantrone, while disagreed regarding anthracyclines. Boukhettala et al.[30] and Hogan et al.[31] found that antimetabolites were the most commonly implicated chemotherapeutic agents with development of NE. Diarrhea induced by cytotoxic compounds is most likely due to mucositis, but may also be due to the alteration of the bacterial flora of the gut.[32]
There were no significant differences in laboratory finding between episodes with or without NE apart from hyponatremia and hypokalemia that were associated with NE, which may be attributed to the marked morbidity and prolonged course of diarrhea in patients with NE. Aksoy et al.[4] found no significant electrolyte abnormalties, which may be explained by the different sample age as Aksoy et al. carried their study in adults who may have higher ability to withstand diarrheal sequlae. The different modes of chemotherapeutic combinations in the two studies may be another factor.
Stool culture was positive in 75% of the NE patients, with Candida being the most commonly revealed organism, followed by Klebsiella, which were both significantly associated with NE, while E. coli was most common in the other group, followed by Salmonella and Shigella. Aksoy et al.[4] in his adult population found Candida to be the most common organism, followed by Klebsiella in cases of NE. Cartoni et al.[6] found a great proportion of Candida and Gram negative bacteria in their patients.
Parasitic infestations should also be considered in the diagnostic work-up of diarrhea in certain settings; they were detected in four diarrheal episodes (giardiasis) among patients in group 2.
Blood culture was positive in all cases of NE, with Klebsiella and Staph. aureus being the most commonly revealed organisms, followed by E. coli, Enterococci, Candida and Serratia orodrifera. Klebsiella, E. coli and Serratia orodrifera were significantly associated with NE. This was some what similar to the results of McCarville et al.,[21] who found E. coli, Klebsiella, Enterococcus, Staphylococcus and Streptococcus species to be the most incriminated organisms. Dietrich et al.[27] had 57% positive blood cultures in patients with NE while Cardona Zorrilla et al.[33] had only 51% positive blood cultures.
Abdominal ultrasonography using a high-resolution probe was the preferred imaging technique in our institution as a screening for NE. Bowel wall thickness ≥3 mm, bowel dilatation, bowel wall irregularity, pneumatosis intestinalis and mucosal enhancement were all significantly associated with NE. McCarville et al.[34] suggested using the cut-off value for bowel wall thickness of ≥3 mm on US to increase both the sensitivity and the accuracy of diagnosis of NE.
Finally, the unavailability of tests for viral etiologies in the stool specimens is considered a limitation in our study as diseases like Cytomegalovirus enterocolitis, which may resemble NE, may be overlooked.
CONCLUSION
This study revealed that diarrhea is a common finding in neutropenic cancer children. Gram negative bacteria and Candida species were the most commonly incriminated organisms. Duration and severity of neutropenia carry a great risk for the development of NE. NE should be highly anticipated during the induction phase of chemotheraputics. Fever, abdominal pain and tenderness are considered red alerts for the oncologists in any neutropenic cancer children with diarrhea.
It is likely that the application of strict, quantitative imaging criteria together with the use of clinical signs and improved antibiotic therapy can reduce the rate of NE-associated morbidity and mortality. Even in the absence of typical clinical findings, the diagnosis of NE can be made when clinical suspicion is confirmed with imaging.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.


 PDF
PDF  Views
Views  Share
Share

