Differential Toxicities of Tyrosine Kinase Inhibitors in the Management of Metastatic Lung Cancer
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(01): 15-17
DOI: DOI: 10.4103/0971-5851.203502
Abstract
Introduction: Erlotinib and gefitinib are the most commonly used epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) in the treatment of EGFR mutant nonsmall cell lung cancer (NSCLC). Both erlotinib and gefitinib have shown equal efficacy in terms of response rates and overall survival. Hence, their toxicity profile becomes the most important determining factor in choosing these agents when treating EGFR mutant NSCLC. In this study, we compared the toxicity profile of erlotinib and gefitinib among an Indian subset of lung cancer patients. Materials and Methods: In this prospective nonrandomized study, 85 patients of South Indian origin with NSCLC were tested for EGFR mutation status, and EGFR mutant patients were started on either erlotinib or gefitinib. They were periodically monitored for drug toxicities. Results: Out of the 85 patients tested, 34 patients were positive for EGFR mutation. Eleven of them were started on erlotinib and 23 were started on gefitinib. The most common side effect of TKIs was skin rash. Nine out of the 11 patients started on erlotinib and 7 of the 23 patients started on gefitinib had skin rash. Grade 3 and 4 skin rash was significantly more among patients treated with erlotinib which resulted in treatment delays. Other side effects of TKIs such as diarrhea and deranged liver functions were similar among the both subsets of patients. Conclusion: Skin toxicity is the major and serious side effect with erlotinib among Indian patients with EGFR mutant lung cancer. This resulted in significant treatment delay, which might adversely affect the overall survival of patients. Gefitinib was better tolerated and had a safer toxicity profile compared to erlotinib in Indian patients.
Keywords
Epidermal growth factor receptor-tyrosine kinase inhibitor - skin toxicity - treatment delayPublication History
Article published online:
06 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Introduction:
Erlotinib and gefitinib are the most commonly used epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) in the treatment of EGFR mutant nonsmall cell lung cancer (NSCLC). Both erlotinib and gefitinib have shown equal efficacy in terms of response rates and overall survival. Hence, their toxicity profile becomes the most important determining factor in choosing these agents when treating EGFR mutant NSCLC. In this study, we compared the toxicity profile of erlotinib and gefitinib among an Indian subset of lung cancer patients.
Materials and Methods:
In this prospective nonrandomized study, 85 patients of South Indian origin with NSCLC were tested for EGFR mutation status, and EGFR mutant patients were started on either erlotinib or gefitinib. They were periodically monitored for drug toxicities.
Results:
Out of the 85 patients tested, 34 patients were positive for EGFR mutation. Eleven of them were started on erlotinib and 23 were started on gefitinib. The most common side effect of TKIs was skin rash. Nine out of the 11 patients started on erlotinib and 7 of the 23 patients started on gefitinib had skin rash. Grade 3 and 4 skin rash was significantly more among patients treated with erlotinib which resulted in treatment delays. Other side effects of TKIs such as diarrhea and deranged liver functions were similar among the both subsets of patients.
Conclusion:
Skin toxicity is the major and serious side effect with erlotinib among Indian patients with EGFR mutant lung cancer. This resulted in significant treatment delay, which might adversely affect the overall survival of patients. Gefitinib was better tolerated and had a safer toxicity profile compared to erlotinib in Indian patients.
Introduction
Epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) have become the standard of care in the management of EGFR mutant lung cancers. Compared to chemotherapeutic agents, EGFR-TKIs have proven their superiority in terms of survival and toxicity profile when treating nonsmall cell lung cancer (NSCLC) patients positive for EGFR mutation.[1,2,3,4]
With the exception of leptomeningeal metastasis where erlotinib has shown better response than gefitinib, both of these TKIs are equally efficient when treating EGFR mutant NSCLC.[5,6] Hence, safer toxicity profile becomes one of the most important factors when choosing these TKIs. In this study, we compared the different toxicity profiles of erlotinib and gefitinib among Indian population.
Materials and Methods
Eighty-five patients of South Indian origin were screened for EGFR mutation, at Cancer Institute, Chennai, India. EGFR mutation test was performed by scorpion probe-based amplified refractory mutation system-reverse transcription-polymerase chain reaction. Patients were started on either erlotinib 150 mg or gefitinib 250 mg in the first-line setting based on physician discretion. Patients were followed up for every month till disease progression. Detailed history and physical examination with special emphasis on drug toxicity was performed at every visit. Toxicity of TKIs was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events v 4.0.[7]
Results
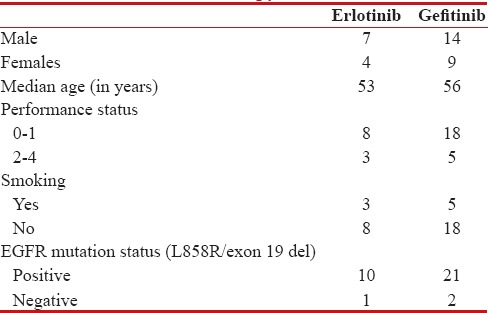
Of the 85 patients tested for EGFR mutation, 34 (40%) patients were positive for the same. Twenty-three patients were started on gefitinib and 11 patients were started on erlotinib. Demographic profile of patients started on TKIs is shown in Table 1.
Table 1
Demograpphic profile of patients on TKI's therapy
|
Skin toxicity was the major side effect of TKIs. Nine of the 11 patients treated with erlotinib had skin toxicity compared to 7 of the 23 patients treated with gefitinib. Grade 3–4 skin toxicity was observed in five patients among erlotinib arm compared to only one patient among gefitinib arm. Of the nine patients who developed skin rash with erlotinib, four required dose reduction from 150 mg to 100 mg. In four patients, erlotinib was changed to gefitinib, as reducing the dose did not result in decrease in skin toxicities [Figure 1]. Gefitinib-induced drug rash was managed conservatively with antihistamines and clindamycin skin ointment without treatment interruption.
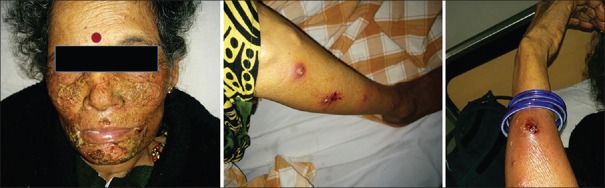
| Figure 1:Erlotinib-induced pustular skin lesions affecting face, leg, and hand
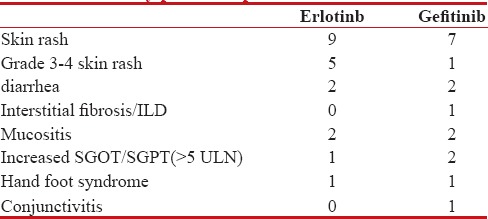
Other side effects such as diarrhea and deranged liver function were comparable in both the groups as shown in Table 2.
Table 2
Toxicity profile of patients treated with TKI's
|
Discussion
Randomized studies have clearly shown survival benefit of TKIs compared to chemotherapy when treating EGFR mutated lung cancer.[1,2,3,4] TKIs such as erlotinib and gefitinib used in the treatment of EGFR mutated lung cancer have similar toxicity profiles, but the grades and severity of the toxicities have not been studied extensively. In this prospective study, we compared toxicity profile of erlotinib and gefitinib in Indian patients.
In our study, skin toxicity was the most important side effect with erlotinib compared to gefitinib. Nine of the 11 patients treated with erlotinib had skin toxicity with five of them having Grade 3–4 skin rash (45.45%) whereas only 7 of the 23 patients treated with gefitinib had skin toxicity and only one among them had Grade 3–4 skin rash. Grade 3–4 toxicity due to erlotinib in our study was much higher than that found in OPTIMAL and EURTAC studies (2% and 13%, respectively).[3,4] This probably is because the steady-state plasma trough concentration by erlotinib at its maximal tolerated dose of 150 mg was 3.5 times higher than that produced by gefitinib at its approved dose of 250 mg once daily which was approximately one-third of the maximum tolerated dose.[8,9]
Because of the skin toxicity, erlotinib dose was reduced to 100 mg in four patients which they could tolerate, and for the other four patients, erlotinib was changed to gefitinib as their skin toxicity recurred with the same severity even following dose reduction. All these four patients tolerated gefitinib well and three of them had Grade 1 rash. In only one patient, erlotinib was continued at 150 mg after treating skin rash with antihistamines and clindamycin topical ointment. There was a significant delay in the treatment in erlotinib arm due to skin toxicity. Treatment had to be stopped for at least 20 days in six of the patients until the rash subsided.
In the gefitinib arm, there were no treatment delays and Grade 2–3 rash was managed with antihistamines and clindamycin topical ointments.
Other side effects such as diarrhea, deranged liver function test, and hand-foot syndrome were comparable in both arms.
The limitation of our study is small sample size. This study can be taken as a pilot study for planning bigger randomized studies.
Conclusion
Skin toxicity is a major side effect with erlotinib among Indian patients which results in significant treatment delay, which in turn may adversely affect the survival of patients with EGFR mutant lung cancer. Dose reduction and changing the drug were helpful in patients who could not tolerate 150 mg of erlotinib. Gefitinib had a much more friendly toxicity profile and was well tolerated among Indian patients.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8.
- Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8.
- Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): A multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42.
- Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46.
- Wu WS, Chen YM, Tsai CM, Shih JF, Chiu CH, Chou KT, et al. Erlotinib has better efficacy than gefitinib in adenocarcinoma patients without EGFR-activating mutations, but similar efficacy in patients with EGFR-activating mutations. Exp Ther Med 2012;3:207-13.
- Lee E, Keam B, Kim DW, Kim TM, Lee SH, Chung DH, et al. Erlotinib versus gefitinib for control of leptomeningeal carcinomatosis in non-small-cell lung cancer. J Thorac Oncol 2013;8:1069-74.
- National Institutes of Health. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE); Version 4.0 Published: May 28, 2009 (v4.03: June 14, 2010). 2010. Available from: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. [Last accessed on 2016 Jun 7].
- Li J, Karlsson MO, Brahmer J, Spitz A, Zhao M, Hidalgo M, et al. CYP3A phenotyping approach to predict systemic exposure to EGFR tyrosine kinase inhibitors. J Natl Cancer Inst 2006;98:1714-23.
- Tan AR, Yang X, Hewitt SM, Berman A, Lepper ER, Sparreboom A, et al. Evaluation of biologic end points and pharmacokinetics in patients with metastatic breast cancer after treatment with erlotinib, an epidermal growth factor receptor tyrosine kinase inhibitor. J Clin Oncol 2004;22:3080-90.

| Figure 1:Erlotinib-induced pustular skin lesions affecting face, leg, and hand
References
- Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8.
- Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8.
- Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): A multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42.
- Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46.
- Wu WS, Chen YM, Tsai CM, Shih JF, Chiu CH, Chou KT, et al. Erlotinib has better efficacy than gefitinib in adenocarcinoma patients without EGFR-activating mutations, but similar efficacy in patients with EGFR-activating mutations. Exp Ther Med 2012;3:207-13.
- Lee E, Keam B, Kim DW, Kim TM, Lee SH, Chung DH, et al. Erlotinib versus gefitinib for control of leptomeningeal carcinomatosis in non-small-cell lung cancer. J Thorac Oncol 2013;8:1069-74.
- National Institutes of Health. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE); Version 4.0 Published: May 28, 2009 (v4.03: June 14, 2010). 2010. Available from: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. [Last accessed on 2016 Jun 7].
- Li J, Karlsson MO, Brahmer J, Spitz A, Zhao M, Hidalgo M, et al. CYP3A phenotyping approach to predict systemic exposure to EGFR tyrosine kinase inhibitors. J Natl Cancer Inst 2006;98:1714-23.
- Tan AR, Yang X, Hewitt SM, Berman A, Lepper ER, Sparreboom A, et al. Evaluation of biologic end points and pharmacokinetics in patients with metastatic breast cancer after treatment with erlotinib, an epidermal growth factor receptor tyrosine kinase inhibitor. J Clin Oncol 2004;22:3080-90.


 PDF
PDF  Views
Views  Share
Share

