Epidemiological Review: Esophagus Squamous Cell Carcinoma in India
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2022; 43(05): 393-403
DOI: DOI: 10.1055/s-0042-1755445
Abstract
Worldwide the incidence of esophagus squamous cell carcinoma (ESCC), remains one of the most common causes of cancer death. ESCC is one of the leading types of cancer in the North and Northeast regions of India among both genders. Risk factors of ESCC include tobacco, alcohol, areca nut, hot beverages, low fruit diet, poor oral hygiene, unpiped water, and human papillomavirus infection. This review tries to elaborate on various modifiable risk factors for ESCC, which have been studied worldwide and need to be studied in India. PubMed was used as a search platform using keywords, such as “esophagus cancer,” “esophagus squamous cell carcinoma,” “epidemiology,” “India,” “incidence,” “mortality,” “risk factors,” “treatment,” “survival,” “prevention” and their corresponding Medical Subject Heading terms, were used in combination with Boolean operators “OR” and “AND.” Studies from India are mostly hospital-based case-control studies from the North region. Further research is required in India to understand the etiology, to design large-scale screening and prevention strategies.
Keywords
esophagus squamous cell carcinoma - esophagus cancer - review - epidemiology - risk factor - IndiaPublication History
Article published online:
20 October 2022
© 2022. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Worldwide the incidence of esophagus squamous cell carcinoma (ESCC), remains one of the most common causes of cancer death. ESCC is one of the leading types of cancer in the North and Northeast regions of India among both genders. Risk factors of ESCC include tobacco, alcohol, areca nut, hot beverages, low fruit diet, poor oral hygiene, unpiped water, and human papillomavirus infection. This review tries to elaborate on various modifiable risk factors for ESCC, which have been studied worldwide and need to be studied in India. PubMed was used as a search platform using keywords, such as “esophagus cancer,” “esophagus squamous cell carcinoma,” “epidemiology,” “India,” “incidence,” “mortality,” “risk factors,” “treatment,” “survival,” “prevention” and their corresponding Medical Subject Heading terms, were used in combination with Boolean operators “OR” and “AND.” Studies from India are mostly hospital-based case-control studies from the North region. Further research is required in India to understand the etiology, to design large-scale screening and prevention strategies.
Keywords
esophagus squamous cell carcinoma - esophagus cancer - review - epidemiology - risk factor - IndiaIntroduction
Esophagus squamous cell carcinoma (ESCC) contributes a significant 90% of the total esophagus cancer (EC) cases worldwide which is an aggressive condition with poor prognosis and low survival rates.[1] Moreover, within the high-incidence region of ESCC, like South America, Africa, Iran, and Asia, the etiologies vary.[2] Consumption of alternate sorts of tobacco, alcohol, hot beverages, low fruit or vegetable diet, processed food, unpiped water, maintaining poor oral hygiene, and human papillomavirus (HPV) infection are the few possible risk factors currently being explored for ESCC worldwide. ESCC has been a major health concern in Kashmir valley and Northeastern states of India where risk factors presently are understudied. This review corroborates the necessity for conducting large-scale epidemiological studies in India to elucidate the risk factors associated with ESCC. Additionally, this review expresses the necessity of presenting the EC burden data on the basis of histological subtypes, considering the paucity of knowledge in this format, and also viewing the vast differences in their etiologies.[2]
Materials and Methods
Advanced search option of the PubMed database was used with the keywords such as “esophagus cancer,” “esophagus squamous cell carcinoma,” “epidemiology,” “India,” “incidence,” “mortality,” “risk factors,” “treatment,” “survival,” “prevention,” and their corresponding Medical Subject Heading terms were used in combination, like “AND” and “OR,” to find published studies on ESCC. This review study was conducted on studies published in English, from the year 2008 to 2020 on ESCC. We excluded animal model studies, studies other than on ESCC, commentaries, clinical or observational veterinary study, and clinical trial studies. Relevant data on descriptive epidemiology and risk factors were explored using databases such as the National Health Portal of India (NHP), Central Water Commission, India (CWC), National Family Health Survey India (NHFS), National Centre for Disease Informatics and Research–Indian Council of Medical Research (NCDIR-ICMR) India, National Cancer Registry Program (NCRP) of India, Census India 2011, National Health Mission India (NHM), International Agency for Research on Cancer (IARC) Monographs, World Health Organization (WHO) guidelines, Global Cancer Observatory 2020, and Cancer Incidence in five continents XI vol. IARC (CI5 XI).
Descriptive Epidemiology
Worldwide, 604,100 new cases and 544,076 cancer death were estimated for EC in the year 2020.[3] India ranks second in EC incidence trailing China which has the highest incidence of EC. In India, it is the fifth most common cancer type in males and the sixth most common cancer type in females. In India, the number of incident cases of esophageal cancer in 2020 was 63,180 out of which 40,183 were males and 22,997 females, and the prevalent cases were 68,607.[3] The male-to-female ratio in India is 2.4:1.[4] ESCC is the most common histological subtype among all cancer registries in India. The top five cancer registries having the highest incidence rate of ESCC are Mizoram, Kamrup Urban, Cachar, Sikkim, and Tripura registries.[5] As per the hospital-based cancer registry report, the esophagus was the leading site in KMIO—Bangalore, AMC—Dibrugarh, BBCI—Guwahati, and in PGIMER—Chandigarh among 35 to 64-year-old males.[6] However, the observed incident cases in the year 2020 have already exceeded the predicted number of incident cases for the year 2035, showing a significant rise in the incident rates.[7]
Survival Data
A study from Jammu, India, suggested that the frequency of survival in ESCC patients is lowered by intake of red chili, snuff, and smoking.[8] Studies from China and Brazil show factors such as gender, marital status, occupation, family history of any cancer, tumor topographical site, differentiation status, and pathological reports, are independent risk factors affecting the overall survival of EC.[9] [10] Other factors such as, weight loss (kg), and body mass index (BMI) variation (kg/m2) predict the stage at diagnosis in the ESCC.[10] The Surveillance, Epidemiology, and End Results (SEER) report of 18 regions from the year 2002 to 2008 for 5-year relative survival in EC continues to be low at around 16.9%.[11] The overall survival of EC is 5 to 30% as stated by the ICMR report. The disease is mostly detected at a stage where it is inoperable in most patients (70–80%), and with an expected survival of 7 to 12 months.
Risk Factors
Recent developments and finding in epidemiological studies have led to the identification of many risk factors associated with ESCC worldwide which need to be studied in India. [Fig. 1].
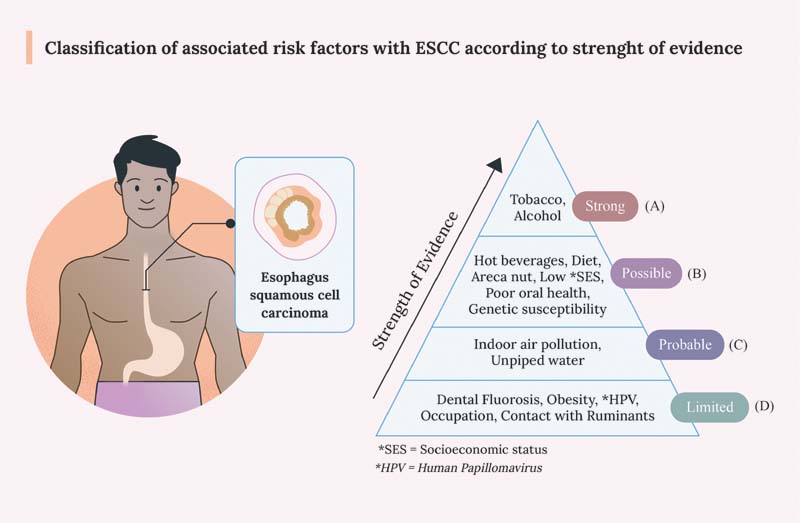
| Figure 1:Classification of associated risk factors with ESCC according to strength of evidence. (A) Strong: risk factors having strongest association as per literature are mentioned in this group followed by (B) Possibly associated risk factors, (C) Probably associated risk factors, and (D) risk factors having limited evidence. ESCC, esophagus squamous cell carcinoma
Strongly Associated
Tobacco Use
Exposure to tobacco smoke and chewing tobacco products has been associated with ESCC. Case-control studies in India have seen a two- to seven-fold increase in the risk of ESSC for chewing tobacco, betel quid with tobacco having a dose–response relationship.[12] In India, the risk of developing tobacco-related cancer was found highest in the Northeastern region with maximum risk found for EC in women.[13] Hospital-based studies from India suggest an increase in the risk of ESCC by smoking tobacco, in the form of cigarettes, bidi, and hookah.[14] [15] [16] Habits such as consumption of nass, snuff, paan chewers, and betel quid chewers also increase the risk of ESCC.[14] [15] [17] Along with cigarettes, other forms of tobacco smoking such as hookah pipes, and cigars exhibit similar risks on ESCC. A study from India on secondhand smoking and ESCC risk reported odds ratio (OR) of 1.32 in exclusive secondhand smokers (never tobacco users), and OR of 3.41 in second-hand smokers who are active chewers, suggesting additive effects of tobacco-related carcinogens[16] ([Table 1]). However, there are only a few studies in India evaluating the risk of tobacco chewing and its association with ESCC and adjusting for potential confounders.
|
Exposure |
Risk estimates (95% CI) Case/control |
Study |
Year |
PMID |
Location |
Case type |
Sample size (case/control) |
Study design |
Adjustment factors |
|---|---|---|---|---|---|---|---|---|---|
|
Indian studies–tobacco |
|||||||||
|
Tobacco chewing with other products |
|||||||||
|
Paan chewers with tobacco (more than 20 years) |
OR = 1.5 77/204 |
Ganesh et al[14] |
2009 |
19846360 |
Mumbai |
ESCC |
442/1,628 |
Case control |
Age, gender, residence, and occupation |
|
Nass chewing (ever chewer) |
OR = 2.88 201/192 |
Dar et al[15] |
2012 |
23033008 |
Kashmir |
ESCC |
702/1,663 |
Case control |
Age, ethnicity, religion, place of residence, education level, cumulative use of cigarette, hookah, ever use of bidi, cannabis, gutka, alcohol, daily fruit, and fresh vegetable consumption |
|
Tobacco inhalation |
|||||||||
|
Snuff |
OR = 3.86 136/71 |
Sehgal et al[12] |
2012 |
23107978 |
Jammu |
ESCC |
200/200 |
Case control |
Not mentioned |
|
Chewing products other than tobacco |
|||||||||
|
Betel nut chewer (ever chewer) |
OR = 2.79 68/52 |
Singh et al[17] |
2015 |
26045981 |
Assam |
ESCC |
99/75 |
Case-control |
Not mentioned |
|
Tobacco smoking |
|||||||||
|
Smoking tobacco |
OR = 1.97 110/63 |
Sehgal et al[12] |
2012 |
23107978 |
Jammu |
ESCC |
200/200 |
Case control |
Not mentioned |
|
Cigarette smoking |
OR = 2.0 40/90 |
Ganesh et al[14] |
2009 |
19846360 |
Mumbai |
ESCC |
442/1,628 |
Case control |
Age, gender, residence, and occupation |
|
Bidi smoking |
OR = 1.8 122/252 |
Ganesh et al[14] |
2009 |
19846360 |
Mumbai |
ESCC |
442/1,628 |
Case control |
Age, gender, residence, and occupation |
|
Water pipe tobacco smoking |
|||||||||
|
Hookah (waterpipe tobacco smoking ever users) |
OR = 1.85 420/699 |
Dar et al[15] |
2012 |
23033008 |
Kashmir |
ESCC |
702/1,663 |
Case control |
Age, ethnicity, religion, place of residence, education level, cumulative use of cigarette, hookah, ever use of bidi, cannabis, gutka, alcohol, daily fruit and fresh vegetable consumption |
|
Indian study–Second hand smoking |
|||||||||
|
Secondhand smoker (never tobacco users) |
OR = 1.32 31/60 |
Rafiq et al[16] |
2016 |
26735535 |
Kashmir |
ESCC |
703/1,664 |
Case control |
Age, ethnicity, religion, place of residence, income, gender, education, the wealth score, ever use of alcohol, salt tea consumption, frequency of close contact with animals, house type, cooking fuel, fruit and vegetable intake |
|
Secondhand smoker (tobacco chewers) |
OR = 3.41 15/11 |
Rafiq et al[16] |
2016 |
26735535 |
Kashmir |
ESCC |
703/1,664 |
Case control |
Age, ethnicity, religion, place of residence, income, gender, education, the wealth score, ever use of alcohol, salt tea consumption, frequency of close contact with animals, house type, cooking fuel, fruit and vegetable intake, tobacco smoking and smokeless tobacco use |
|
International studies–tobacco |
|||||||||
|
Cigarette or pipe (ever) |
RR = 1.33 |
Tran et al |
2005 |
15455378 |
China |
ESCC |
1,958 |
Cohort |
Age |
|
Ever smoker |
HR = 1.36 |
Fan et al |
2008 |
18444169 |
Shanghai |
EC |
101 |
Cohort |
Level of education, body mass index, number of drinks consumed per day, number of years of drinking, and summed intakes of preserved food items, fresh fruits, and fresh vegetables |
|
Smoking tobacco index (daily tobacco intake × duration of smoking) overall survival |
HR = 1.21 |
Liu et al |
2020 |
32071596 |
China |
ESCC |
944 |
Cohort |
Multivariate |
|
Smokeless tobacco users |
OR = 2.06–12.8 |
Gupta et al |
2018 |
30264755 |
Eastern Mediterranean |
EC |
80 studies |
Meta-analysis |
|
|
Ex-smokers |
HR = 1.29 |
Cho et al |
2017 |
28973012 |
Korean |
EC |
9,171 |
Cohort |
Age, gender, exercise, income, BMI, diabetes mellitus, and alcohol |
|
Current smokers |
HR = 1.87 |
Cho et al |
2017 |
28973012 |
Korean |
EC |
9,171 |
Cohort |
Age, gender, exercise, income, BMI, diabetes mellitus, and alcohol |
Exposure |
Risk estimates (95% CI) Case/control |
Study |
Year |
PMID |
Location |
Case type |
Sample size (case/control) |
Study design |
Adjustment factors |
|---|---|---|---|---|---|---|---|---|---|
|
Indian studies–alcohol |
|||||||||
|
Alcohol |
OR = 1.8 66/131 |
Ganesh et al[14] |
2009 |
19846360 |
Mumbai |
ESCC |
442/1,628 |
Case control |
Age, gender, residence and occupation |
|
Alcohol |
OR = 2.21 41/22 |
Singh et al[17] |
2015 |
26045981 |
Assam |
EC |
110/75 |
Case control |
Not mentioned |
|
Zu (local liquor) |
OR = 1.34 32/28 |
Lalpawimawha |
2016 |
Not found |
Mizoram |
EC |
138/276 |
Case control |
Betel quid consumption, tobacco consumption, smoking, BMI at 20 years of age and family history of cancer; education level and income level, dietary habits and physical activity except for each independent variable |
|
Zu (local liquor) + commercial |
OR = 9.82 21/12 |
Lalpawimawha |
2016 |
Not found |
Mizoram |
EC |
138/276 |
Case control |
|
|
International studies–alcohol |
|||||||||
|
Mild-to-moderate drinkers |
HR = 1.52 |
Cho et al |
2017 |
28973012 |
Korean |
EC |
5,839 |
Cohort |
Age, gender, exercise, income, BMI, diabetes mellitus, and smoking status |
|
Heavy drinkers |
HR = 3.13 |
Cho et al |
2017 |
28973012 |
Korean |
EC |
5,839 |
Cohort |
Age, gender, exercise, income, BMI, diabetes mellitus, and smoking status |
|
Light drinker |
RR = 1.25 |
Islami et al[20] |
2011 |
21190191 |
Iran, Italy, France |
ESSC |
16 studies |
Systematic review and meta-analysis |
Systematic review and meta-analysis |
|
Moderate drinker |
RR = 2.32 |
Islami et al[20] |
2011 |
21190191 |
Iran, Italy, France |
ESSC |
27 studies |
Systematic review and meta-analysis |
Systematic review and meta-analysis |
|
Heavy drinkers |
RR = 5.38 |
Islami et al[20] |
2011 |
21190191 |
Iran, Italy, France |
ESSC |
20 studies |
Systematic review and meta-analysis |
Systematic review and meta-analysis |
|
Exposure |
Risk estimates (95% CI) |
Study |
Year |
PMID |
Location |
Case type |
Sample size |
Study design |
|---|---|---|---|---|---|---|---|---|
|
International study–areca nut |
||||||||
|
Areca nut |
OR = 3.05 |
Akhtar et al[25] |
2013 |
23224324 |
Kuwait |
ESCC |
12 case-control study |
Meta-analysis |
|
Areca nut and tobacco smoking |
OR = 6.79 |
Akhtar et al[25] |
2013 |
23224324 |
Kuwait |
ESCC |
6 case-control study |
Meta-analysis |
|
Exposure |
Risk estimates (95% CI) Case | control |
Study |
Year |
PMID |
Location |
Case type |
Sample size (case/control) |
Study design |
Adjustment factors |
|---|---|---|---|---|---|---|---|---|---|
|
Indian studies–poor oral health |
|||||||||
|
Cleaning of teeth with brush |
OR = 0.11 Case | Control 50/528 |
Dar et al[28] |
2013 |
23900216 |
India |
ESCC |
703/1,664 |
Case control |
Age, ethnicity, residence, education, wealth score, fruit and vegetable intake, bidi smoking, gutka chewing, alcohol consumption and cumulative use of hookah, cigarette, and nass |
|
Cleaning of teeth with finger |
OR = 0.51 Case | Control 488/957 |
Dar et al[28] |
2013 |
23900216 |
India |
ESCC |
703/1664 |
Case control |
|
|
International studies–poor oral health |
|||||||||
|
Frequency of brushing teeth <= 1 |
OR = 1.81 486/510 |
Chen et al |
2017 |
27778330 |
China |
ESCC |
616/770 |
Case control |
Age, gender, education, marital status, tobacco smoking, alcohol drinking, tea drinking, family history of ESCC, daily consumption of pickled vegetables, daily consumption of fresh fruits, and wealth score |
|
≥6 tooth loss (after age 20) |
OR = 1.48 266/330 |
Chen et al |
2017 |
27778330 |
China |
ESCC |
616/770 |
Case control |
|
|
Excessive tooth loss (≥12 excess tooth loss) |
HR = 1.66 |
Sheikh et al[26] |
2019 |
30611753 |
Northeastern Iran |
ESCC |
50,045 individuals |
Cohort |
Age, gender, residence counties, ethnicity, quartiles of the socioeconomic status, opium consumption through smoking, opium consumption through ingestion, drinking hot tea at ≥60°C, daily intake of fruits, daily intake of vegetables, drinking un-piped water, indoor air pollution, daily contact with ruminants, alcohol drinking, cigarette smoking, nass chewing |
|
Mswaki stick |
OR = 1.7 133/75 |
Menya et al[27] |
2019 |
30582155 |
Africa |
ESCC |
430/440 |
Case control |
Gender, ethnicity, alcohol and tobacco, alcohol intensity, beverage drinking, family history of EC, and continuous: age, education score, tooth brushing frequency + brush type + DMFT (not for lost/decayed teeth), leukoplakia, dental fluorosis |
|
No. of missing teeth ≥ 6 |
OR = 1.3 87/55 |
Menya et al[27] |
2019 |
30582155 |
Africa |
ESCC |
430/440 |
Case control |
|
|
No. of decayed teeth ≥ 3 |
OR = 4.4 131 | 37 |
Menya et al[27] |
2019 |
30582155 |
Africa |
ESCC |
430/440 |
Case control |
|
|
DMFT count ≥ 8 |
OR = 3.0 133/54 |
Menya et al[27] |
2019 |
30582155 |
Africa |
ESCC |
430/440 |
Case control |
|
|
Frequency of brushing teeth (never) tobacco users–fully adjusted |
OR = 2.53 222/324 |
Abnet et al. |
2009 |
18990747 |
Golestan, Iran |
ESCC |
283/560 |
Case control |
Age, gender, place of residence, ethnicity, alcohol drinking, use of tobacco, opium, or both, education in three categories, number of appliances, and fruit and vegetable intake |
|
Frequency of brushing teeth (never) alcoholic beverage drinkers–fully adjusted |
OR = 2.15 222/324 |
Abnet et al. |
2009 |
18990747 |
Golestan, Iran |
ESCC |
283/560 |
Case control |
|
|
Teeth loss |
OR = 1.31 |
Chen et al |
2015 |
26462879 |
China, Iran, Japan, India, the United States, Finland |
ESCC |
6 studies |
Meta analysis |
Not mentioned (Forest's plot) |
|
teeth brushing |
OR = 0.57 |
Chen et al |
2015 |
26462879 |
China, Iran, Japan, India, the United States, Finland |
ESCC |
4 studies |
Meta analysis |
Not mentioned (Forest's plot) |
References
- Yang J, Liu X, Cao S, Dong X, Rao S, Cai K. Understanding esophageal cancer: the challenges and opportunities for the next decade. Front Oncol 2020; 10: 1727
- Hull R, Mbele M, Makhafola T. et al. A multinational review: oesophageal cancer in low to middle-income countries. Oncol Lett 2020; 20 (04) 42
- Cancer today. Accessed March 22, 2021 at: http://gco.iarc.fr/today/home
- Choksi D, Kolhe KM, Ingle M. et al. Esophageal carcinoma: An epidemiological analysis and study of the time trends over the last 20 years from a single center in India. J Family Med Prim Care 2020; 9 (03) 1695-1699
- Bray F, Colombet M, Mery L. et al; World Health Organization. Cancer Incidence in Five Continents, Vol. XI. Accessed March 22, 2021 at: https://publications.iarc.fr/_publications/media/download/6371/8015478d4d1381e2d10b7df95e6762987b34a20e.pdf
- National Centre for Disease Informatics and Research, National Cacer Registry Programme. Consolidated report of hospital based cancer registries: 2012–2014. Accessed March 30, 2021 at: https://ncdirindia.org/ncrp/ALL_NCRP_REPORTS/HBCR_REPORT_2012_2014/index.htm
- National Centre for Disease Informatics and Research, National Cacer Registry Programme. Consolidated report of population based cancer registry: 2012-2014. Accessed March 30, 2021 at: https://ncdirindia.org/ncrp/Annual_Reports.aspx
- Sehgal S, Kaul S, Gupta BB, Dhar MK. Risk factors and survival analysis of the esophageal cancer in the population of Jammu, India. Indian J Cancer 2012; 49 (02) 245-250
- He Y, Liang D, Du L. et al. Clinical characteristics and survival of 5283 esophageal cancer patients: A multicenter study from eighteen hospitals across six regions in China. Cancer Commun (Lond) 2020; 40 (10) 531-544
- Tustumi F, Kimura CMS, Takeda FR. et al. Prognostic factors and survival analysis in esophageal carcinoma. Arq Bras Cir Dig 2016; 29 (03) 138-141
- Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol 2013; 19 (34) 5598-5606
- Gupta P, Arora M, Sinha D, Asma S, Parascandola M. Smokeless Tobacco and Public Health in India. Ministry of Health & Family Welfare, Government of India. New Delhi, India: Govt. of India; 2016
- Asthana S, Patil RS, Labani S. Tobacco-related cancers in India: a review of incidence reported from population-based cancer registries. Indian J Med Paediatr Oncol 2016; 37 (03) 152-157
- Ganesh B, Talole SD, Dikshit R. Tobacco, alcohol and tea drinking as risk factors for esophageal cancer: a case-control study from Mumbai, India. Cancer Epidemiol 2009; 33 (06) 431-434
- Dar N, Bhat G, Shah I. et al. Hookah smoking, nass chewing, and oesophageal squamous cell carcinoma in Kashmir, India. Br J Cancer 2012; 107 (09) 1618-1623
- Rafiq R, Shah IA, Bhat GA. et al. Secondhand smoking and the risk of esophageal squamous cell carcinoma in a high incidence region, kashmir, india: a case-control-observational study. Medicine (Baltimore) 2016; 95 (01) e2340
- Singh V, Singh LC, Singh AP. et al. Status of epigenetic chromatin modification enzymes and esophageal squamous cell carcinoma risk in northeast Indian population. Am J Cancer Res 2015; 5 (03) 979-999
- IARC. Alcohol Drinking: IARC Monographs on the Evaluation of the Carcinogenic Risks to Humans Volume 44. Accessed March 31, 2021 at: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Alcohol-Drinking-1988
- Kim MK, Ko MJ, Han JT. Alcohol consumption and mortality from all-cause and cancers among 1.34 million Koreans: the results from the Korea national health insurance corporation's health examinee cohort in 2000. Cancer Causes Control 2010; 21 (12) 2295-2302
- Islami F, Fedirko V, Tramacere I. et al. Alcohol drinking and esophageal squamous cell carcinoma with focus on light-drinkers and never-smokers: a systematic review and meta-analysis. Int J Cancer 2011; 129 (10) 2473-2484
- Chokshi DA, El-Sayed AM, Stine NW. J-shaped curves and public health. JAMA 2015; 314 (13) 1339-1340
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Betel-quid and areca-nut chewing and some areca-nut derived nitrosamines. IARC Monogr Eval Carcinog Risks Hum 2004; 85: 1-334
- Shah G, Chaturvedi P, Vaishampayan S. Arecanut as an emerging etiology of oral cancers in India. Indian J Med Paediatr Oncol 2012; 33 (02) 71-79
- Chen CH, Lu HI, Wang YM. et al. Areca nut is associated with younger age of diagnosis, poor chemoradiotherapy response, and shorter overall survival in esophageal squamous cell carcinoma. PLoS One 2017; 12 (02) e0172752
- Akhtar S. Areca nut chewing and esophageal squamous-cell carcinoma risk in Asians: a meta-analysis of case-control studies. Cancer Causes Control 2013; 24 (02) 257-265
- Sheikh M, Poustchi H, Pourshams A. et al. Individual and combined effects of environmental risk factors for esophageal cancer based on results from the Golestan cohort study. Gastroenterology 2019; 156 (05) 1416-1427
- ;Menya D, Maina SK, Kibosia C. et al. Dental fluorosis and oral health in the African Esophageal Cancer Corridor: Findings from the Kenya ESCCAPE case-control study and a pan-African perspective. Int J Cancer 2019; 145 (01) 99-109
- Dar NA, Islami F, Bhat GA. et al. Poor oral hygiene and risk of esophageal squamous cell carcinoma in Kashmir. Br J Cancer 2013; 109 (05) 1367-1372
- Salunke S, Shah V, Ostbye T. et al. Prevalence of dental caries, oral health awareness and treatment-seeking behavior of elderly population in rural Maharashtra. Indian J Dent Res 2019; 30 (03) 332-336
- Wild CP, Weiderpass E, Stewart BW. World Cancer Report: Cancer Research for Cancer Prevention. Accessed March 22, 2021 at: https://publications.iarc.fr/Non-Series-Publications/World-Cancer-Reports/World-Cancer-Report-Cancer-Research-For-Cancer-Prevention-2020
- Lagergren J, Andersson G, Talbäck M. et al. Marital status, education, and income in relation to the risk of esophageal and gastric cancer by histological type and site. Cancer 2016; 122 (02) 207-212
- Gao P, Yang X, Suo C. et al. Socioeconomic status is inversely associated with esophageal squamous cell carcinoma risk: results from a population-based case-control study in China. Oncotarget 2018; 9 (06) 6911-6923
- Dar NA, Shah IA, Bhat GA. et al. Socioeconomic status and esophageal squamous cell carcinoma risk in Kashmir, India. Cancer Sci 2013; 104 (09) 1231-1236
- Khan NA, Teli MA, Mohib-Ul Haq M, Bhat GM, Lone MM, Afroz F. A survey of risk factors in carcinoma esophagus in the valley of Kashmir, Northern India. J Cancer Res Ther 2011; 7 (01) 15-18
- About the global cancer update programme. Accessed March 24, 2021 at: https://www.wcrf.org/int/continuous-update-project
- IARC. . Some Aromatic Amines, Anthraquinones and Nitroso Compounds, and Inorganic Fluorides Used in Drinking-Water and Dental Preparations. Accessed March 23, 2021 at: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Some-Aromatic-Amines-Anthraquinones-And-Nitroso-Compounds-And-Inorganic-Fluorides-Used-In-Drinking-water-And-Dental-Preparations-1982
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Red Meat and Processed Meat. Lyon (FR): Lyon (FR): International Agency for Research on Cancer; 2018
- Steevens J, Schouten LJ, Goldbohm RA, van den Brandt PA. Vegetables and fruits consumption and risk of esophageal and gastric cancer subtypes in the Netherlands Cohort Study. Int J Cancer 2011; 129 (11) 2681-2693
- Lin S, Wang X, Huang C. et al. Consumption of salted meat and its interactions with alcohol drinking and tobacco smoking on esophageal squamous-cell carcinoma. Int J Cancer 2015; 137 (03) 582-589
- Golozar A, Etemadi A, Kamangar F. et al. Food preparation methods, drinking water source, and esophageal squamous cell carcinoma in the high-risk area of Golestan, Northeast Iran. Eur J Cancer Prev 2016; 25 (02) 123-129
- Hashemian M, Poustchi H, Abnet CC. et al. Dietary intake of minerals and risk of esophageal squamous cell carcinoma: results from the Golestan Cohort Study. Am J Clin Nutr 2015; 102 (01) 102-108
- Narzary Y, Brahma J, Brahma C, Das S. A study on indigenous fermented foods and beverages of Kokrajhar, Assam, India. Journal of Ethnic Foods 2016; 3 (04) 284-291
- Loomis D, Guyton KZ, Grosse Y. et al; International Agency for Research on Cancer Monograph Working Group. Carcinogenicity of drinking coffee, mate, and very hot beverages. Lancet Oncol 2016; 17 (07) 877-878
- ;IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Drinking Coffee, Mate, and Very Hot Beverages. Lyon (FR): International Agency for Research on Cancer; 2018.
- Islami F, Pourshams A, Nasrollahzadeh D. et al. Tea drinking habits and oesophageal cancer in a high risk area in northern Iran: population based case-control study. BMJ 2009; 338: b929
- Yu C, Tang H, Guo Y. et al; China Kadoorie Biobank Collaborative Group. Hot tea consumption and its interactions with alcohol and tobacco use on the risk for esophageal cancer: a population-based cohort study. Ann Intern Med 2018; 168 (07) 489-497
- Lubin JH, De Stefani E, Abnet CC. et al. Maté drinking and esophageal squamous cell carcinoma in South America: pooled results from two large multicenter case-control studies. Cancer Epidemiol Biomarkers Prev 2014; 23 (01) 107-116
- Dar NA, Bhat GA, Shah IA. et al. Salt tea consumption and esophageal cancer: a possible role of alkaline beverages in esophageal carcinogenesis. Int J Cancer 2015; 136 (06) E704 –E710
- WHO. . Fuel for life: household energy and health. Accessed March 23, 2021 at: https://www.who.int/publications/i/item/9789241563161
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. IARC Monogr Eval Carcinog Risks Hum 2010; 92: 1-853
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Some drinking-water disinfectants and contaminants, including arsenic. IARC Monogr Eval Carcinog Risks Hum 2004; 84 (01) 1-477
- ;Talagala IA, Nawarathne M, Arambepola C. Novel risk factors for primary prevention of oesophageal carcinoma: a case-control study from Sri Lanka. BMC Cancer 2018; 18 (01) 1135
- National Health Portal of India, Gateway to Authentic Health Information. . Health tips. Accessed April 7, 2021 at: https://www.nhp.gov.in/
- Sanikini H, Muller DC, Sophiea M. et al. Anthropometric and reproductive factors and risk of esophageal and gastric cancer by subtype and subsite: Results from the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Int J Cancer 2020; 146 (04) 929-942
- Sanikini H, Muller DC, Chadeau-Hyam M, Murphy N, Gunter MJ, Cross AJ. Anthropometry, body fat composition and reproductive factors and risk of oesophageal and gastric cancer by subtype and subsite in the UK Biobank cohort. PLoS One 2020; 15 (10) e0240413
- Khairnar MR, Dodamani AS, Jadhav HC, Naik RG, Deshmukh MA. Mitigation of fluorosis - a review. J Clin Diagn Res 2015; 9 (06) ZE05-ZE09
- Chaudhry M, Prabhakar I, Gupta B, Anand R, Sehrawat P, Thakar SS. Prevalence of dental fluorosis among adolescents in schools of Greater Noida, Uttar Pradesh. Journal of Indian Association of Public Health Dentistry 2017; 15 (01) 36
- Kotecha PV, Patel SV, Bhalani KD, Shah D, Shah VS, Mehta KG. Prevalence of dental fluorosis & dental caries in association with high levels of drinking water fluoride content in a district of Gujarat, India. Indian J Med Res 2012; 135 (06) 873-877
- Podgorski JE, Labhasetwar P, Saha D, Berg M. Prediction modeling and mapping of groundwater fluoride contamination throughout India. Environ Sci Technol 2018; 52 (17) 9889-9898
- Lee CP, Lee YH, Lian IB, Su CC. Increased prevalence of esophageal cancer in areas with high levels of nickel in farm soils. J Cancer 2016; 7 (12) 1724-1730
- Zafarzadeh A, Rahimzadeh H, Mahvi AH. Health risk assessment of heavy metals in vegetables in an endemic esophageal cancer region in Iran. Health Scope 2018; 7 (03) ): DOI: 10.5812/jhealthscope.12340.
- Ministry of Jal Shakti, Department of Water Resources, River Development and Ganga Rejuvenation, GoI.. Central Water Commission. Accessed March 27, 2021 at: http://www.cwc.gov.in/
- Zhang SK, Guo LW, Chen Q. et al. The association between human papillomavirus 16 and esophageal cancer in Chinese population: a meta-analysis. BMC Cancer 2015; 15: 1096
- Yong F, Xudong N, Lijie T. Human papillomavirus types 16 and 18 in esophagus squamous cell carcinoma: a meta-analysis. Ann Epidemiol 2013; 23 (11) 726-734
- Shah I, Bhat G, Rafiq R. et al. Strenuous occupational physical activity: Potential association with esophageal squamous cell carcinoma risk. Proceedings of Singapore Healthcare 2019; 28: 201010581986086
- Dar NA, Islami F, Bhat GA. et al. Contact with animals and risk of oesophageal squamous cell carcinoma: outcome of a case-control study from Kashmir, a high-risk region. Occup Environ Med 2014; 71 (03) 208-214
- Nasrollahzadeh D, Ye W, Shakeri R. et al. Contact with ruminants is associated with esophageal squamous cell carcinoma risk. Int J Cancer 2015; 136 (06) 1468-1474
- Islami F, Kamangar F, Nasrollahzadeh D. et al. Socio-economic status and oesophageal cancer: results from a population-based case-control study in a high-risk area. Int J Epidemiol 2009; 38 (04) 978-988
- IARC. . Fruit and Vegetables: IARC Handbooks of Cancer Prevention Volume 8. Accessed March 24, 2021 at: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Handbooks-Of-Cancer-Prevention/Fruit-And-Vegetables-2003
- Jankowski JAZ, de Caestecker J, Love SB. et al; AspECT Trial Team. Esomeprazole and aspirin in Barrett's oesophagus (AspECT): a randomised factorial trial. Lancet 2018; 392 (10145): 400-408
- Etemadi A, Golozar A, Kamangar F. et al. Large body size and sedentary lifestyle during childhood and early adulthood and esophageal squamous cell carcinoma in a high-risk population. Ann Oncol 2012; 23 (06) 1593-1600
- GATS2 (Global Adult Tobacco Survey) Fact Sheet, India, 2016–17. Accessed July 20, 2022 at: https://www.tobaccofreekids.org/assets/global/pdfs/en/GATS_India_2016-17_FactSheet.pdf
- National Family Health Survey. Accessed April 5, 2021 at: http://rchiips.org/nfhs/factsheet_NFHS-4.shtml
Address for correspondence
Rajesh Dikshit, PhDDirector room, 2nd Floor, Department of Molecular Epidemiology and Population Genetics, Centre for Cancer Epidemiology, Actrec CampusSector 22, near Owe village, Navi Mumbai, 410210, MaharashtraIndiaEmail: dixr24@hotmail.comPublication History
Article published online:
20 October 2022© 2022. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Figure 1:Classification of associated risk factors with ESCC according to strength of evidence. (A) Strong: risk factors having strongest association as per literature are mentioned in this group followed by (B) Possibly associated risk factors, (C) Probably associated risk factors, and (D) risk factors having limited evidence. ESCC, esophagus squamous cell carcinoma
References
- Yang J, Liu X, Cao S, Dong X, Rao S, Cai K. Understanding esophageal cancer: the challenges and opportunities for the next decade. Front Oncol 2020; 10: 1727
- Hull R, Mbele M, Makhafola T. et al. A multinational review: oesophageal cancer in low to middle-income countries. Oncol Lett 2020; 20 (04) 42
- Cancer today. Accessed March 22, 2021 at: http://gco.iarc.fr/today/home
- Choksi D, Kolhe KM, Ingle M. et al. Esophageal carcinoma: An epidemiological analysis and study of the time trends over the last 20 years from a single center in India. J Family Med Prim Care 2020; 9 (03) 1695-1699
- Bray F, Colombet M, Mery L. et al; World Health Organization. Cancer Incidence in Five Continents, Vol. XI. Accessed March 22, 2021 at: https://publications.iarc.fr/_publications/media/download/6371/8015478d4d1381e2d10b7df95e6762987b34a20e.pdf
- National Centre for Disease Informatics and Research, National Cacer Registry Programme. Consolidated report of hospital based cancer registries: 2012–2014. Accessed March 30, 2021 at: https://ncdirindia.org/ncrp/ALL_NCRP_REPORTS/HBCR_REPORT_2012_2014/index.htm
- National Centre for Disease Informatics and Research, National Cacer Registry Programme. Consolidated report of population based cancer registry: 2012-2014. Accessed March 30, 2021 at: https://ncdirindia.org/ncrp/Annual_Reports.aspx
- Sehgal S, Kaul S, Gupta BB, Dhar MK. Risk factors and survival analysis of the esophageal cancer in the population of Jammu, India. Indian J Cancer 2012; 49 (02) 245-250
- He Y, Liang D, Du L. et al. Clinical characteristics and survival of 5283 esophageal cancer patients: A multicenter study from eighteen hospitals across six regions in China. Cancer Commun (Lond) 2020; 40 (10) 531-544
- Tustumi F, Kimura CMS, Takeda FR. et al. Prognostic factors and survival analysis in esophageal carcinoma. Arq Bras Cir Dig 2016; 29 (03) 138-141
- Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol 2013; 19 (34) 5598-5606
- Gupta P, Arora M, Sinha D, Asma S, Parascandola M. Smokeless Tobacco and Public Health in India. Ministry of Health & Family Welfare, Government of India. New Delhi, India: Govt. of India; 2016
- Asthana S, Patil RS, Labani S. Tobacco-related cancers in India: a review of incidence reported from population-based cancer registries. Indian J Med Paediatr Oncol 2016; 37 (03) 152-157
- Ganesh B, Talole SD, Dikshit R. Tobacco, alcohol and tea drinking as risk factors for esophageal cancer: a case-control study from Mumbai, India. Cancer Epidemiol 2009; 33 (06) 431-434
- Dar N, Bhat G, Shah I. et al. Hookah smoking, nass chewing, and oesophageal squamous cell carcinoma in Kashmir, India. Br J Cancer 2012; 107 (09) 1618-1623
- Rafiq R, Shah IA, Bhat GA. et al. Secondhand smoking and the risk of esophageal squamous cell carcinoma in a high incidence region, kashmir, india: a case-control-observational study. Medicine (Baltimore) 2016; 95 (01) e2340
- Singh V, Singh LC, Singh AP. et al. Status of epigenetic chromatin modification enzymes and esophageal squamous cell carcinoma risk in northeast Indian population. Am J Cancer Res 2015; 5 (03) 979-999
- IARC. Alcohol Drinking: IARC Monographs on the Evaluation of the Carcinogenic Risks to Humans Volume 44. Accessed March 31, 2021 at: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Alcohol-Drinking-1988
- Kim MK, Ko MJ, Han JT. Alcohol consumption and mortality from all-cause and cancers among 1.34 million Koreans: the results from the Korea national health insurance corporation's health examinee cohort in 2000. Cancer Causes Control 2010; 21 (12) 2295-2302
- Islami F, Fedirko V, Tramacere I. et al. Alcohol drinking and esophageal squamous cell carcinoma with focus on light-drinkers and never-smokers: a systematic review and meta-analysis. Int J Cancer 2011; 129 (10) 2473-2484
- Chokshi DA, El-Sayed AM, Stine NW. J-shaped curves and public health. JAMA 2015; 314 (13) 1339-1340
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Betel-quid and areca-nut chewing and some areca-nut derived nitrosamines. IARC Monogr Eval Carcinog Risks Hum 2004; 85: 1-334
- Shah G, Chaturvedi P, Vaishampayan S. Arecanut as an emerging etiology of oral cancers in India. Indian J Med Paediatr Oncol 2012; 33 (02) 71-79
- Chen CH, Lu HI, Wang YM. et al. Areca nut is associated with younger age of diagnosis, poor chemoradiotherapy response, and shorter overall survival in esophageal squamous cell carcinoma. PLoS One 2017; 12 (02) e0172752
- Akhtar S. Areca nut chewing and esophageal squamous-cell carcinoma risk in Asians: a meta-analysis of case-control studies. Cancer Causes Control 2013; 24 (02) 257-265
- Sheikh M, Poustchi H, Pourshams A. et al. Individual and combined effects of environmental risk factors for esophageal cancer based on results from the Golestan cohort study. Gastroenterology 2019; 156 (05) 1416-1427
- ;Menya D, Maina SK, Kibosia C. et al. Dental fluorosis and oral health in the African Esophageal Cancer Corridor: Findings from the Kenya ESCCAPE case-control study and a pan-African perspective. Int J Cancer 2019; 145 (01) 99-109
- Dar NA, Islami F, Bhat GA. et al. Poor oral hygiene and risk of esophageal squamous cell carcinoma in Kashmir. Br J Cancer 2013; 109 (05) 1367-1372
- Salunke S, Shah V, Ostbye T. et al. Prevalence of dental caries, oral health awareness and treatment-seeking behavior of elderly population in rural Maharashtra. Indian J Dent Res 2019; 30 (03) 332-336
- Wild CP, Weiderpass E, Stewart BW. World Cancer Report: Cancer Research for Cancer Prevention. Accessed March 22, 2021 at: https://publications.iarc.fr/Non-Series-Publications/World-Cancer-Reports/World-Cancer-Report-Cancer-Research-For-Cancer-Prevention-2020
- Lagergren J, Andersson G, Talbäck M. et al. Marital status, education, and income in relation to the risk of esophageal and gastric cancer by histological type and site. Cancer 2016; 122 (02) 207-212
- Gao P, Yang X, Suo C. et al. Socioeconomic status is inversely associated with esophageal squamous cell carcinoma risk: results from a population-based case-control study in China. Oncotarget 2018; 9 (06) 6911-6923
- Dar NA, Shah IA, Bhat GA. et al. Socioeconomic status and esophageal squamous cell carcinoma risk in Kashmir, India. Cancer Sci 2013; 104 (09) 1231-1236
- Khan NA, Teli MA, Mohib-Ul Haq M, Bhat GM, Lone MM, Afroz F. A survey of risk factors in carcinoma esophagus in the valley of Kashmir, Northern India. J Cancer Res Ther 2011; 7 (01) 15-18
- About the global cancer update programme. Accessed March 24, 2021 at: https://www.wcrf.org/int/continuous-update-project
- IARC. . Some Aromatic Amines, Anthraquinones and Nitroso Compounds, and Inorganic Fluorides Used in Drinking-Water and Dental Preparations. Accessed March 23, 2021 at: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Some-Aromatic-Amines-Anthraquinones-And-Nitroso-Compounds-And-Inorganic-Fluorides-Used-In-Drinking-water-And-Dental-Preparations-1982
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Red Meat and Processed Meat. Lyon (FR): Lyon (FR): International Agency for Research on Cancer; 2018
- Steevens J, Schouten LJ, Goldbohm RA, van den Brandt PA. Vegetables and fruits consumption and risk of esophageal and gastric cancer subtypes in the Netherlands Cohort Study. Int J Cancer 2011; 129 (11) 2681-2693
- Lin S, Wang X, Huang C. et al. Consumption of salted meat and its interactions with alcohol drinking and tobacco smoking on esophageal squamous-cell carcinoma. Int J Cancer 2015; 137 (03) 582-589
- Golozar A, Etemadi A, Kamangar F. et al. Food preparation methods, drinking water source, and esophageal squamous cell carcinoma in the high-risk area of Golestan, Northeast Iran. Eur J Cancer Prev 2016; 25 (02) 123-129
- Hashemian M, Poustchi H, Abnet CC. et al. Dietary intake of minerals and risk of esophageal squamous cell carcinoma: results from the Golestan Cohort Study. Am J Clin Nutr 2015; 102 (01) 102-108
- Narzary Y, Brahma J, Brahma C, Das S. A study on indigenous fermented foods and beverages of Kokrajhar, Assam, India. Journal of Ethnic Foods 2016; 3 (04) 284-291
- Loomis D, Guyton KZ, Grosse Y. et al; International Agency for Research on Cancer Monograph Working Group. Carcinogenicity of drinking coffee, mate, and very hot beverages. Lancet Oncol 2016; 17 (07) 877-878
- ;IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Drinking Coffee, Mate, and Very Hot Beverages. Lyon (FR): International Agency for Research on Cancer; 2018.
- Islami F, Pourshams A, Nasrollahzadeh D. et al. Tea drinking habits and oesophageal cancer in a high risk area in northern Iran: population based case-control study. BMJ 2009; 338: b929
- Yu C, Tang H, Guo Y. et al; China Kadoorie Biobank Collaborative Group. Hot tea consumption and its interactions with alcohol and tobacco use on the risk for esophageal cancer: a population-based cohort study. Ann Intern Med 2018; 168 (07) 489-497
- Lubin JH, De Stefani E, Abnet CC. et al. Maté drinking and esophageal squamous cell carcinoma in South America: pooled results from two large multicenter case-control studies. Cancer Epidemiol Biomarkers Prev 2014; 23 (01) 107-116
- Dar NA, Bhat GA, Shah IA. et al. Salt tea consumption and esophageal cancer: a possible role of alkaline beverages in esophageal carcinogenesis. Int J Cancer 2015; 136 (06) E704 –E710
- WHO. . Fuel for life: household energy and health. Accessed March 23, 2021 at: https://www.who.int/publications/i/item/9789241563161
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. IARC Monogr Eval Carcinog Risks Hum 2010; 92: 1-853
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Some drinking-water disinfectants and contaminants, including arsenic. IARC Monogr Eval Carcinog Risks Hum 2004; 84 (01) 1-477
- ;Talagala IA, Nawarathne M, Arambepola C. Novel risk factors for primary prevention of oesophageal carcinoma: a case-control study from Sri Lanka. BMC Cancer 2018; 18 (01) 1135
- National Health Portal of India, Gateway to Authentic Health Information. . Health tips. Accessed April 7, 2021 at: https://www.nhp.gov.in/
- Sanikini H, Muller DC, Sophiea M. et al. Anthropometric and reproductive factors and risk of esophageal and gastric cancer by subtype and subsite: Results from the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Int J Cancer 2020; 146 (04) 929-942
- Sanikini H, Muller DC, Chadeau-Hyam M, Murphy N, Gunter MJ, Cross AJ. Anthropometry, body fat composition and reproductive factors and risk of oesophageal and gastric cancer by subtype and subsite in the UK Biobank cohort. PLoS One 2020; 15 (10) e0240413
- Khairnar MR, Dodamani AS, Jadhav HC, Naik RG, Deshmukh MA. Mitigation of fluorosis - a review. J Clin Diagn Res 2015; 9 (06) ZE05-ZE09
- Chaudhry M, Prabhakar I, Gupta B, Anand R, Sehrawat P, Thakar SS. Prevalence of dental fluorosis among adolescents in schools of Greater Noida, Uttar Pradesh. Journal of Indian Association of Public Health Dentistry 2017; 15 (01) 36
- Kotecha PV, Patel SV, Bhalani KD, Shah D, Shah VS, Mehta KG. Prevalence of dental fluorosis & dental caries in association with high levels of drinking water fluoride content in a district of Gujarat, India. Indian J Med Res 2012; 135 (06) 873-877
- Podgorski JE, Labhasetwar P, Saha D, Berg M. Prediction modeling and mapping of groundwater fluoride contamination throughout India. Environ Sci Technol 2018; 52 (17) 9889-9898
- Lee CP, Lee YH, Lian IB, Su CC. Increased prevalence of esophageal cancer in areas with high levels of nickel in farm soils. J Cancer 2016; 7 (12) 1724-1730
- Zafarzadeh A, Rahimzadeh H, Mahvi AH. Health risk assessment of heavy metals in vegetables in an endemic esophageal cancer region in Iran. Health Scope 2018; 7 (03) ): DOI: 10.5812/jhealthscope.12340.
- Ministry of Jal Shakti, Department of Water Resources, River Development and Ganga Rejuvenation, GoI.. Central Water Commission. Accessed March 27, 2021 at: http://www.cwc.gov.in/
- Zhang SK, Guo LW, Chen Q. et al. The association between human papillomavirus 16 and esophageal cancer in Chinese population: a meta-analysis. BMC Cancer 2015; 15: 1096
- Yong F, Xudong N, Lijie T. Human papillomavirus types 16 and 18 in esophagus squamous cell carcinoma: a meta-analysis. Ann Epidemiol 2013; 23 (11) 726-734
- Shah I, Bhat G, Rafiq R. et al. Strenuous occupational physical activity: Potential association with esophageal squamous cell carcinoma risk. Proceedings of Singapore Healthcare 2019; 28: 201010581986086
- Dar NA, Islami F, Bhat GA. et al. Contact with animals and risk of oesophageal squamous cell carcinoma: outcome of a case-control study from Kashmir, a high-risk region. Occup Environ Med 2014; 71 (03) 208-214
- Nasrollahzadeh D, Ye W, Shakeri R. et al. Contact with ruminants is associated with esophageal squamous cell carcinoma risk. Int J Cancer 2015; 136 (06) 1468-1474
- Islami F, Kamangar F, Nasrollahzadeh D. et al. Socio-economic status and oesophageal cancer: results from a population-based case-control study in a high-risk area. Int J Epidemiol 2009; 38 (04) 978-988
- IARC. . Fruit and Vegetables: IARC Handbooks of Cancer Prevention Volume 8. Accessed March 24, 2021 at: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Handbooks-Of-Cancer-Prevention/Fruit-And-Vegetables-2003
- Jankowski JAZ, de Caestecker J, Love SB. et al; AspECT Trial Team. Esomeprazole and aspirin in Barrett's oesophagus (AspECT): a randomised factorial trial. Lancet 2018; 392 (10145): 400-408
- Etemadi A, Golozar A, Kamangar F. et al. Large body size and sedentary lifestyle during childhood and early adulthood and esophageal squamous cell carcinoma in a high-risk population. Ann Oncol 2012; 23 (06) 1593-1600
- GATS2 (Global Adult Tobacco Survey) Fact Sheet, India, 2016–17. Accessed July 20, 2022 at: https://www.tobaccofreekids.org/assets/global/pdfs/en/GATS_India_2016-17_FactSheet.pdf
- National Family Health Survey. Accessed April 5, 2021 at: http://rchiips.org/nfhs/factsheet_NFHS-4.shtml


 PDF
PDF  Views
Views  Share
Share

