Extensive Metastases in Prostatic Carcinoma with Normal Prostate-Specific Antigen and Raised Carcinoembryonic Antigen – Small Cell Carcinoma of Prostate: A Rare Entity
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2018; 39(04): 539-542
DOI: DOI: 10.4103/ijmpo.ijmpo_72_17
Abstract
Small cell carcinoma of prostate is a neuroendocrine tumor of prostate seen in 0.5%–2% of men with carcinoma prostate. Prostate-specific antigen (PSA) is a common tumor marker which is often raised in prostatic carcinoma. However, prostatic carcinoma can progress with normal or low serum PSA levels at the time of diagnosis. Carcinoembryonic antigen (CEA) is a tumor marker of different carcinomas. Small cell carcinoma prostate is a highly aggressive tumor which can progress with normal or low serum PSA levels and raised CEA levels. We report a case of 65-year-old male with enlarged prostate with extra-prostatic spread, hepatic metastases, metastatic retroperitoneal and pelvic lymph nodes, osteoblastic metastasis in lumbar spine with normal serum PSA, and raised CEA levels. Prostatic biopsy was suggestive of small cell carcinoma.
Keywords
Carcinoembryonic antigen - neuroendocrine tumor - prostate-specific antigen - prostatic carcinoma - small cell carcinomaPublication History
Article published online:
17 June 2021
© 2018. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Small cell carcinoma of prostate is a neuroendocrine tumor of prostate seen in 0.5%–2% of men with carcinoma prostate. Prostate-specific antigen (PSA) is a common tumor marker which is often raised in prostatic carcinoma. However, prostatic carcinoma can progress with normal or low serum PSA levels at the time of diagnosis. Carcinoembryonic antigen (CEA) is a tumor marker of different carcinomas. Small cell carcinoma prostate is a highly aggressive tumor which can progress with normal or low serum PSA levels and raised CEA levels. We report a case of 65-year-old male with enlarged prostate with extra-prostatic spread, hepatic metastases, metastatic retroperitoneal and pelvic lymph nodes, osteoblastic metastasis in lumbar spine with normal serum PSA, and raised CEA levels. Prostatic biopsy was suggestive of small cell carcinoma.
Keywords
Carcinoembryonic antigen - neuroendocrine tumor - prostate-specific antigen - prostatic carcinoma - small cell carcinomaIntroduction
Prostatic carcinoma can be acinar and nonacinar. Acinar prostatic carcinoma constitutes 90% of prostatic carcinoma. Nonacinar carcinoma constitutes 5%–10% of prostatic carcinomas. They are sarcomatoid carcinoma, ductal adenocarcinoma, urothelial carcinoma, squamous and adenosquamous carcinoma, basal cell carcinoma, and neuroendocrine tumors, specifically small-cell carcinoma. Prostate-specific antigen (PSA) is a common tumor marker which is increased in Ca prostate. However, approximately 1/3rd of men with Ca prostate do not have raised serum PSA levels at time of diagnosis.[1] Ca prostate can progress despite low-serum PSA levels. Carcinoembryonic antigen (CEA) and CA 19-9 though tumor markers in different carcinomas can be raised in Ca prostate. CEA can be elevated in patients with androgen-independent prostate Ca. Small cell carcinoma of the prostate is aggressive malignancy of the prostate contributing to approximately 0.5%–2% of men with carcinoma prostate.
Case Report
A 65-year-old male patient presented with increased frequency of micturition associated with burning micturition for 15 days with significant weight loss and loss of appetite for 3 months. Ultrasonography revealed mild hepatomegaly with multiple target lesions and bilateral hydronephrosis and hydroureter with overdistended urinary bladder. Multiple enlarged retroperitoneal discrete lymph nodes in pre- and para-aortic, inter aorto-caval, pre- and retro-caval, right common and internal iliac region were noted. The prostate was enlarged with heterogeneous echotexture with irregular prostatic outlines, especially along its right lateral margin. Prostatic weight was approximately 74 g. There was an invasion of bladder base and involvement of right ureterovesical junction.
Postcontrast computed tomography of the abdomen and pelvis revealed multiple discrete variable sized round to oval hypodense lesions in both lobes of the liver which were better delineated against adjacent enhancing hepatic parenchyma in portal and venous phases, suggestive of metastases [Figure 1a]. Multiple well-defined discrete retroperitoneal lymph nodes showing heterogeneous enhancement of variable sizes (1–3 cm) were noted in pre- and para-aortic, retro-aortic, pre- and retro-caval, bilateral common iliac and right internal iliac and right obturator regions [Figure 1b], [c], [d]. Prostate was enlarged in size measuring approximately 58 (CC) × 64 (T) × 45 (AP) mm. It showed lobulated margins and heterogeneous density on plain study with heterogeneous postcontrast enhancement. It was elevating urinary bladder base with bladder invasion measuring approximately 53 mm × 12 mm. Acute angle between posterior bladder wall and right seminal vesicle was obscured. Right seminal vesicle was enlarged measuring 52 mm × 22 mm suggestive of involvement. Soft tissue density lesion was noted in periprostatic and pararectal region suggestive of extraprostatic spread with rectal involvement [Figure 2]. Mild hydronephrosis with hydroureter was noted due to involvement of right ureterovesical junction.
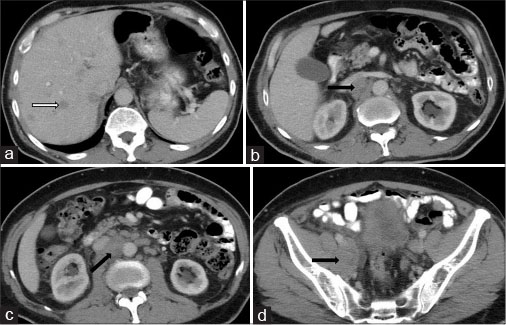
| Figure 1:A 65-year-old male complaining of increased frequency of micturition, anorexia, and weight loss due to small cell carcinoma of prostate. Contrast-enhanced computed tomography image of abdomen and pelvis showing multiple hypodense lesions in both hepatic lobes suggestive of metastases (white arrow) (a), multiple retroperitoneal lymph nodes in pre- and para-aortic, interaortocaval, retrocaval, right internal iliac region (black arrows) (b-d)
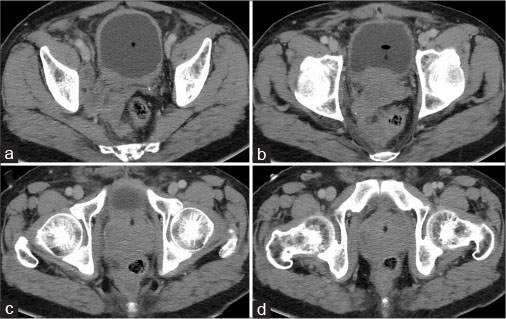
| Figure.2:A 65-year-old male complaining of increased frequency of micturition, anorexia, and weight loss due to small cell carcinoma of prostate contrast-enhanced computed tomography image of abdomen and pelvis showing thickened right seminal vesicle and right obturator lymph nodes (white arrow) (a), thickened seminal vesicle (white arrow head), spread in right peri-rectal fat and bladder invasion (curved white arrow) (b), enlarged prostate with heterogeneous density with spread in right peri-rectal space (black arrows) (c and d)
MRI lumbosacral spine with pelvis revealed multiple well-defined variable sized round to oval lesions in lumbar vertebrae, sacrum and pelvic bones, appearing hypointense on T1-weighted image and T2-weighted image (T2WI) and hyperintense on Short tau inversion recovery (STIR) [Figure 3]. Ill-defined T2WI hyperintense fluid collection was noted in the retroperitoneum on right side likely lymphedema due to lymphatic obstruction. Prostate was enlarged with hypointense lesion in peripheral zone with extra-prostatic spread on right side with involvement and thickening of right seminal vesicle with extension in right perirectal fat [Figure 4] and [5].
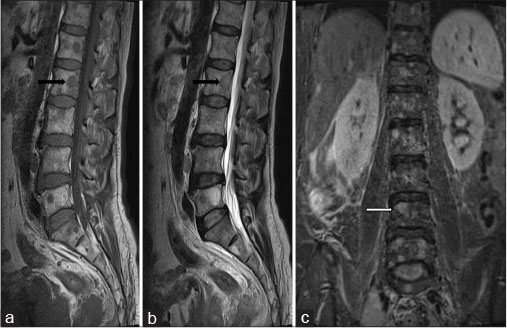
| Figure.3:A 65-year-old male complaining of increased frequency of micturition, anorexia, and weight loss due to small cell carcinoma of prostate. Magnetic resonance imaging of lumbosacral spine (a) sagittal T1-weighted, (b) Sagittal T2-weighted, (c) coronal STIR – multiple hypointense lesions on T1-weighted and T2-weighted (black arrows) appearing hyperintense on STIR (white arrow) - suggestive of osteoblastic metastases
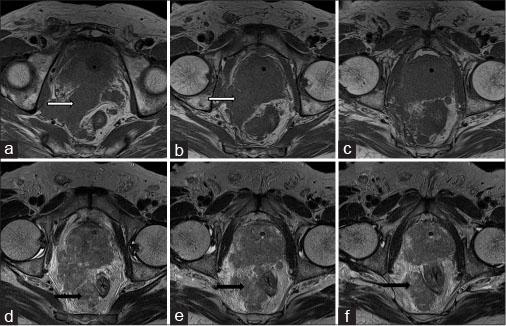
| Figure.4:A 65-year-old male complaining of increased frequency of micturition, anorexia, and weight loss due to small cell carcinoma of prostate. Axial T1-weighted (a-c) and T2-weighted (d-f) showing enlarged prostate with extra-prostatic spread on right side with involvement of right seminal vesicle (white arrow) and peri-rectal fat (black arrow)

| Figure.5:65-year-old male complaining of increased frequency of micturition, anorexia and weight loss due to small cell carcinoma of prostate. Sagittal T2-weighted showing enlarged prostate with urinary bladder invasion (black arrow) and extra-prostatic spread on right side with involvement of right peri-rectal fat (white arrow)
Serum PSA level was 1.418 ng/ml (normal range for age 60–69 years: ≤4.5 ng/ml). CEA level was 11.15 ng/ml (normal range: 0–5 ng/ml).
Prostatic biopsy was suggestive of small cell carcinoma [Figure 6].

| Figure.6:(H and E, ×400) showing multiple small round neoplastic cells (black arrow) with high nuclear to cytoplasmic ratio and scanty cytoplasm with hyperchromatic nuclei suggestive of small cell carcinoma
Discussion
PSA is a protein produced by the prostate gland. Its elevation in the blood occurs due to disruption of prostate cellular architecture with a resultant hike into circulation. PSA is specific to prostatic tissue and hence is elevated in CA prostate, Benign Prostatic Hyperplasia (BPH), prostatitis, prostatic manipulations (such as prostate examination, catheter placement, ejaculation, prostatic biopsy). CA prostate is the most common carcinoma in male patients but the second leading cause of cancer death due to the slow course of disease.[2]
Histopathological grading and PSA levels are used as prognostic factors in Ca prostate. There is a proven correlation between tumor volume and stage and PSA levels. Although PSA is a common tumor marker for Ca prostate, approximately 1/3rd of men with Ca prostate do not have raised serum PSA levels at time of diagnosis.[2] Prostate Ca is usually androgen-dependent tumor. It is sensitive to androgen deprivation therapy (ADT) in advanced metastatic stage. The PSA level is extremely useful in monitoring diseased status and its treatment response. Recurrence of Ca prostate can be diagnosed on rising PSA levels even after antihormonal therapy. Rarely, Ca prostate can progress despite low serum PSA levels. Leibovici et al. reported that patients with metastatic Ca prostate and a low serum PSA account for <1 href="https://www.thieme-connect.com/products/ejournals/html/10.4103/ijmpo.ijmpo_72_17#JR_3" xss=removed>3] Yamamoto et al. reported 8 patients with progression of Ca prostate (Stage M1) in the presence of undetectable or low serum PSA levels.[4] Most of these patients had rapid Ca progression with poor prognosis. ADT was ineffective in these cases.
CEA and CA 19-9 are tumor markers in different carcinomas and not of Ca prostate. CEA is a membrane-associated glycoprotein that is expressed in gut epithelium and is raised in gastrointestinal Ca and Ca breast.[5]
Ghazizadeh et al. found that normal prostatic tissue can produce CEA and can be raised in Ca prostate using immunochemical study.[6] Feuer et al. documented that CEA is elevated in patients with androgen-independent prostate Ca, also CEA had no prognostic effect on length of survival.[7] CA 19-9 (carbohydrate antigen 19-9) is a tumor marker of gastrointestinal CA. It is also found in normal prostate and seminal plasma. Till now, no data is available in role of CA 19-9 in cases of Ca prostate.[5] Ca prostate usually is an androgen-dependent tumor. Even advanced cases are initially sensitive of anti-hormonal treatment. Nearly, all cases after some time progress to androgen-independent CA. These results in challenge for clinicians as chemotherapy show limited efficacy. In metastatic situation, median time to progression is 5 months in hormone-independent Ca prostate.[2] Serum CEA, CA19-9, and CA 15-3 levels are raised in patients with metastatic prostate. Progressive androgen independent prostate cancer with low serum PSA level was characterised by high proportion of lytic bone disease, visceral metastases, sensitivity to cisplatin based chemotherapy and small cell or poorly differentiated prostate cancer on histology.[8] [9] As ADT is ineffective in advanced metastatic Ca prostate; Yamamoto et al. recommended initial therapy with a combination of radiotherapy and systemic chemotherapy.[4] Patients with prostate carcinoma progression and undetectable serum PSA levels after radical prostatectomy can have rapid progression.[5]
Neuroendocrine tumors of prostate are a heterogenous group that includes conventional adenocarcinoma of the prostate with focal differentiation, small cell carcinoma, large cell neuroendocrine carcinoma, carcinoid tumors, tumors with Paneth cell-like neuroendocrine differentiation.[10] [11]
Small cell carcinoma of the prostate is aggressive malignancy of the prostate and is an entity, contributing to approximately 0.5%–2% of men with carcinoma prostate.[10] It was described by Wenk et al. about 30 years ago. It is a spectrum of anaplastic tumor of the prostate. It is usually associated with conventional prostatic adenocarcinoma. It usually occurs in patients with high-grade adenocarcinoma, which are treated with ADT. Small cell carcinoma usually does not benefit from ADT.[10] Small cell carcinoma of prostate shows a distinct clinical, radiological, and biological behaviour. It is characterized by extensive local disease, visceral metastases, and normal or low serum PSA levels despite extensive metastases. It shows rapid progression, markedly enlarged prostate, osteolytic bone metastases, and low PSA relative to disease burden. The patient usually presents with voiding and neurological symptoms such as sensory or motor deficit, confusion, or other constitutional symptoms. It usually arises in peripheral zone and tends to invade outside the prostate and surrounding organs with metastases in lymph nodes and distant organs, more commonly than conventional prostatic adenocarcinoma. Thus, marked prostatic enlargement with extensive local and distant spread, normal PSA levels, and paraneoplastic syndrome should raise suspicion of small cell carcinoma of the prostate. About 10% of small cell carcinomas of prostate secrete hormones such as ACTH or ADH who present with paraneoplastic syndrome. They tend to invade through the prostatic capsule and involve periprostatic soft tissue, seminal vesicles, urinary bladder wall, and rectum. They tend to metastasize to pre- and para-aortic and pelvic lymph nodes, bones, liver, adrenals, lungs, and brain. As they are more aggressive, they typically present at a higher pathologic stage as compared to conventional adenocarcinoma of the prostate. Prognosis of prostatic small cell carcinoma is poor with a median survival of less than a year. They generally do not respond to hormonal or radiation therapy and also surgical alternative is not curative. They are treated with a regimen of cisplatin and etoposide.[11]
Conclusion
Prostate Ca may progress in spite of low serum PSA levels. Although CEA and CA 19-9 are not prostate Ca specific, their levels might be raised in aggressive/advanced prostatic Ca. Their role in Ca prostate is uncertain. This case is unique as the patient had advanced metastatic Ca prostate with normal serum PSA levels and high-serum CEA and Ca 19-9 levels. It is suggestive of aggressive variant of Ca prostate with rapid progression and poor prognosis.
Conflict of Interest
There are no conflicts of interest.
References
- Mikuz G. Histologic classification of prostate cancer. Anal Quant Cytopathol Histpathol 2015; 37: 39-47
- Brandes A, Weinmann M, Reichmann U, Becker G, Gärtner HV, Bamberg M. Serum elevation of CEA and CA 19-9 in an aggressive variant of prostatic carcinoma. Acta Oncol 2001; 40: 879-81
- Leibovici D, Spiess PE, Agarwal PK, Tu SM, Pettaway CA, Hitzhusen K. et al. Prostate cancer progression in the presence of undetectable or low serum prostate-specific antigen level. Cancer 2007; 109: 198-204
- Yamamoto S, Ito T, Akiyama A, Aizawa T, Miki M, Tachibana M. M1 prostate cancer with a serum level of prostate-specific antigen less than 10 ng/mL. Int J Urol 2001; 8: 374-9
- Juang GD, Hwang TI, Wang YH. Metastatic prostate cancer with elevated serum levels of CEA and CA19-9. Urol Sci 2014; 25: 28-30
- Ghazizadeh M, Kagawa S, Izumi K, Maebayashi K, Takigawa H, Saiki T. et al. Immunohistochemical detection of carcinoembryonic antigen in benign hyperplasia and adenocarcinoma of the prostate with monoclonal antibody. J Urol 1984; 131: 501-3
- Feuer JA, Lush RM, Venzon D, Duray P, Tompkins A, Sartor O. et al. Elevated carcinoembryonic antigen in patients with androgen-independent prostate cancer. J Investig Med 1998; 46: 66-72
- Nishio R, Furuya Y, Nagakawa O, Fuse H. Metastatic prostate cancer with normal level of serum prostate-specific antigen. Int Urol Nephrol 2003; 35: 189-92
- Sella A, Konichezky M, Flex D, Sulkes A, Baniel J. Low PSA metastatic androgen- independent prostate cancer. Eur Urol 2000; 38: 250-4
- Nadal R, Schweizer M, Kryvenko ON, Epstein JI, Eisenberger MA. Small cell carcinoma of the prostate. Nat Rev Urol 2014; 11: 213-9
- Rubenstein JH, Katin MJ, Mangano MM, Dauphin J, Salenius SA, Dosoretz DE. et al. Small cell anaplastic carcinoma of the prostate: Seven new cases, review of the literature, and discussion of a therapeutic strategy. Am J Clin Oncol 1997; 20: 376-80
Address for correspondence
Publication History
Article published online:
17 June 2021
© 2018. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2,
Noida-201301 UP, India

| Figure 1:A 65-year-old male complaining of increased frequency of micturition, anorexia, and weight loss due to small cell carcinoma of prostate. Contrast-enhanced computed tomography image of abdomen and pelvis showing multiple hypodense lesions in both hepatic lobes suggestive of metastases (white arrow) (a), multiple retroperitoneal lymph nodes in pre- and para-aortic, interaortocaval, retrocaval, right internal iliac region (black arrows) (b-d)

| Figure.2:A 65-year-old male complaining of increased frequency of micturition, anorexia, and weight loss due to small cell carcinoma of prostate contrast-enhanced computed tomography image of abdomen and pelvis showing thickened right seminal vesicle and right obturator lymph nodes (white arrow) (a), thickened seminal vesicle (white arrow head), spread in right peri-rectal fat and bladder invasion (curved white arrow) (b), enlarged prostate with heterogeneous density with spread in right peri-rectal space (black arrows) (c and d)

| Figure.3:A 65-year-old male complaining of increased frequency of micturition, anorexia, and weight loss due to small cell carcinoma of prostate. Magnetic resonance imaging of lumbosacral spine (a) sagittal T1-weighted, (b) Sagittal T2-weighted, (c) coronal STIR – multiple hypointense lesions on T1-weighted and T2-weighted (black arrows) appearing hyperintense on STIR (white arrow) - suggestive of osteoblastic metastases

| Figure.4:A 65-year-old male complaining of increased frequency of micturition, anorexia, and weight loss due to small cell carcinoma of prostate. Axial T1-weighted (a-c) and T2-weighted (d-f) showing enlarged prostate with extra-prostatic spread on right side with involvement of right seminal vesicle (white arrow) and peri-rectal fat (black arrow)

| Figure.5:65-year-old male complaining of increased frequency of micturition, anorexia and weight loss due to small cell carcinoma of prostate. Sagittal T2-weighted showing enlarged prostate with urinary bladder invasion (black arrow) and extra-prostatic spread on right side with involvement of right peri-rectal fat (white arrow)

| Figure.6:(H and E, ×400) showing multiple small round neoplastic cells (black arrow) with high nuclear to cytoplasmic ratio and scanty cytoplasm with hyperchromatic nuclei suggestive of small cell carcinoma
References
- Mikuz G. Histologic classification of prostate cancer. Anal Quant Cytopathol Histpathol 2015; 37: 39-47
- Brandes A, Weinmann M, Reichmann U, Becker G, Gärtner HV, Bamberg M. Serum elevation of CEA and CA 19-9 in an aggressive variant of prostatic carcinoma. Acta Oncol 2001; 40: 879-81
- Leibovici D, Spiess PE, Agarwal PK, Tu SM, Pettaway CA, Hitzhusen K. et al. Prostate cancer progression in the presence of undetectable or low serum prostate-specific antigen level. Cancer 2007; 109: 198-204
- Yamamoto S, Ito T, Akiyama A, Aizawa T, Miki M, Tachibana M. M1 prostate cancer with a serum level of prostate-specific antigen less than 10 ng/mL. Int J Urol 2001; 8: 374-9
- Juang GD, Hwang TI, Wang YH. Metastatic prostate cancer with elevated serum levels of CEA and CA19-9. Urol Sci 2014; 25: 28-30
- Ghazizadeh M, Kagawa S, Izumi K, Maebayashi K, Takigawa H, Saiki T. et al. Immunohistochemical detection of carcinoembryonic antigen in benign hyperplasia and adenocarcinoma of the prostate with monoclonal antibody. J Urol 1984; 131: 501-3
- Feuer JA, Lush RM, Venzon D, Duray P, Tompkins A, Sartor O. et al. Elevated carcinoembryonic antigen in patients with androgen-independent prostate cancer. J Investig Med 1998; 46: 66-72
- Nishio R, Furuya Y, Nagakawa O, Fuse H. Metastatic prostate cancer with normal level of serum prostate-specific antigen. Int Urol Nephrol 2003; 35: 189-92
- Sella A, Konichezky M, Flex D, Sulkes A, Baniel J. Low PSA metastatic androgen- independent prostate cancer. Eur Urol 2000; 38: 250-4
- Nadal R, Schweizer M, Kryvenko ON, Epstein JI, Eisenberger MA. Small cell carcinoma of the prostate. Nat Rev Urol 2014; 11: 213-9
- Rubenstein JH, Katin MJ, Mangano MM, Dauphin J, Salenius SA, Dosoretz DE. et al. Small cell anaplastic carcinoma of the prostate: Seven new cases, review of the literature, and discussion of a therapeutic strategy. Am J Clin Oncol 1997; 20: 376-80


 PDF
PDF  Views
Views  Share
Share

