High-Dose Methotrexate-Induced Dermatological Eruption: A Rare Manifestation of Chemotoxicity
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2022; 43(05): 431-433
DOI: DOI: 10.1055/s-0042-1748939
High-dose methotrexate (HD-MTX) that is given in the form of continuous infusion over 24 hours forms an essential part of extra compartmental therapy pediatric-inspired protocols for treatment of acute lymphoblastic leukemia (ALL). Dermatological eruptions due to HD-MTX are rarely reported as compared with low-dose MTX (LD-MTX) therapy given for immunodermatological disorders. Defects in skin and mucosa pave way for bacterial invasion and sepsis in a neutropenic patient adding up to complications and contributing to morbidity due to ALL.
We report a 24-year-old female diagnosed as Philadelphia positive B cell ALL treated with Berlin-Frank-Munchester-95 protocol, who developed severe methotrexate-induced dermatological eruption post first HD-MTX infusion. She had an uneventful induction course with documented minimal residual disease negativity post induction. After documenting baseline normal complete blood count and biochemistry values, hydration along with alkalinization was started as per institute's protocol. Interacting medications such as dasatinib, septran, 6-mercaptopurine, and folate were stopped. HD-MTX was administered at a dose of 5 gm/m2 as a 24-hour infusion. Her maximum serum level of MTX at 36 hours was 1.38 micromol/L and leucovorin rescue was guided according to Bleyer's nomogram. She was conservatively managed for grade 2 emesis and headache on day 4 of administration. She was discharged on day 6 with balanced pH and soda bicarbonate mouth gargles for mild oral soreness. On day 9, she started developing abdominal pain, bloody stools, with high-grade fever, requiring immediate readmission. She looked sick, febrile, icteric with multiple pruritic plaques on an erythematous base distributed all over the body, palmoplantar erythrodysesthesia, and grade 4 oral mucositis. Her laboratory investigations were suggestive of severe myelosuppression along with grade 2 AKI and grade 3 transaminitis according to Common Terminology Criteria for Adverse Events Version 5.0. She was given bowel rest with total parenteral nutrition, broad spectrum antibiotics, granulocyte colony stimulating factor, transfusion support with red blood cells, and platelets including granulocyte infusion to tide over the gram-negative bacterial sepsis. The skin care included application of topical urease cream with mild corticosteroids for palmoplantar lesions, emollients for generalized skin lesions, and balanced pH gargles for oral mucositis. The skin lesions became hyperkeratotic over time and healed with desquamation as shown in [Fig. 1]. Her general condition improved after an inpatient stay of 14 days with good nursing care.
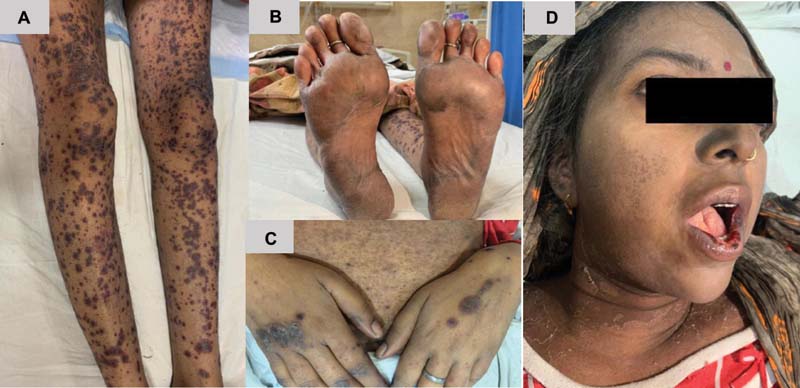
| Figure 1:(A) Pruritic plaques on an erythematous base, (B) plantar erythrodysesthesia, (C) hyperkeratotic plaques over dorsum of hand, (D) oral mucositis and facial skin in healing stage with peeling of epidermis.
Pulse LD-MTX therapy given weekly without leucovorin rescue for psoriasis and rheumatoid arthritis commonly causes dermatological eruptions at a lower threshold than HD-MTX (0.2 vs 1 mM/L).[1] Perifolliculitis, photosensitivity, erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis occur due to immunologic mechanisms from drug presentation by keratinocytes to cytotoxic T lymphocytes.[2] [3] Palmoplantar erythema with bullous lesions result due to concentration of this drug in eccrine glands.[4] Histopathological changes like acanthosis, epidermal necrolysis, interface dermatitis, and dyskeratosis result due to direct toxicity on rapidly multiplying keratinocytes.[5] Dasatinib forms backbone of pH + ALL and is known to delay the proper clearance of methotrexate.[6] Other known risk factors include advanced age, hypoalbuminemia, pre-existing renal dysfunction, and low folate stores in the patient's body. Variations in expression of genes SLCO1B1, BCRP, and ABCB1 along with single nucleotide polymorphisms of various enzymes involved in metabolism of methotrexate like 5,10-methylene-tetrahydrofolate reductase and thymidylate synthetase can predict individual toxicities.[7] Our patient had significant toxicity despite adequate clearance and stopping interacting medications like dasatinib, thus involving genomics into discussion. Ensuring a robust supportive care with the use of dermoprotective agents will surely enhance faster recovery of such patients.
High-dose methotrexate (HD-MTX) that is given in the form of continuous infusion over 24 hours forms an essential part of extra compartmental therapy pediatric-inspired protocols for treatment of acute lymphoblastic leukemia (ALL). Dermatological eruptions due to HD-MTX are rarely reported as compared with low-dose MTX (LD-MTX) therapy given for immunodermatological disorders. Defects in skin and mucosa pave way for bacterial invasion and sepsis in a neutropenic patient adding up to complications and contributing to morbidity due to ALL.
We report a 24-year-old female diagnosed as Philadelphia positive B cell ALL treated with Berlin-Frank-Munchester-95 protocol, who developed severe methotrexate-induced dermatological eruption post first HD-MTX infusion. She had an uneventful induction course with documented minimal residual disease negativity post induction. After documenting baseline normal complete blood count and biochemistry values, hydration along with alkalinization was started as per institute's protocol. Interacting medications such as dasatinib, septran, 6-mercaptopurine, and folate were stopped. HD-MTX was administered at a dose of 5 gm/m2 as a 24-hour infusion. Her maximum serum level of MTX at 36 hours was 1.38 micromol/L and leucovorin rescue was guided according to Bleyer's nomogram. She was conservatively managed for grade 2 emesis and headache on day 4 of administration. She was discharged on day 6 with balanced pH and soda bicarbonate mouth gargles for mild oral soreness. On day 9, she started developing abdominal pain, bloody stools, with high-grade fever, requiring immediate readmission. She looked sick, febrile, icteric with multiple pruritic plaques on an erythematous base distributed all over the body, palmoplantar erythrodysesthesia, and grade 4 oral mucositis. Her laboratory investigations were suggestive of severe myelosuppression along with grade 2 AKI and grade 3 transaminitis according to Common Terminology Criteria for Adverse Events Version 5.0. She was given bowel rest with total parenteral nutrition, broad spectrum antibiotics, granulocyte colony stimulating factor, transfusion support with red blood cells, and platelets including granulocyte infusion to tide over the gram-negative bacterial sepsis. The skin care included application of topical urease cream with mild corticosteroids for palmoplantar lesions, emollients for generalized skin lesions, and balanced pH gargles for oral mucositis. The skin lesions became hyperkeratotic over time and healed with desquamation as shown in [Fig. 1]. Her general condition improved after an inpatient stay of 14 days with good nursing care.

| Figure 1:(A) Pruritic plaques on an erythematous base, (B) plantar erythrodysesthesia, (C) hyperkeratotic plaques over dorsum of hand, (D) oral mucositis and facial skin in healing stage with peeling of epidermis.
Pulse LD-MTX therapy given weekly without leucovorin rescue for psoriasis and rheumatoid arthritis commonly causes dermatological eruptions at a lower threshold than HD-MTX (0.2 vs 1 mM/L).[1] Perifolliculitis, photosensitivity, erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis occur due to immunologic mechanisms from drug presentation by keratinocytes to cytotoxic T lymphocytes.[2] [3] Palmoplantar erythema with bullous lesions result due to concentration of this drug in eccrine glands.[4] Histopathological changes like acanthosis, epidermal necrolysis, interface dermatitis, and dyskeratosis result due to direct toxicity on rapidly multiplying keratinocytes.[5] Dasatinib forms backbone of pH + ALL and is known to delay the proper clearance of methotrexate.[6] Other known risk factors include advanced age, hypoalbuminemia, pre-existing renal dysfunction, and low folate stores in the patient's body. Variations in expression of genes SLCO1B1, BCRP, and ABCB1 along with single nucleotide polymorphisms of various enzymes involved in metabolism of methotrexate like 5,10-methylene-tetrahydrofolate reductase and thymidylate synthetase can predict individual toxicities.[7] Our patient had significant toxicity despite adequate clearance and stopping interacting medications like dasatinib, thus involving genomics into discussion. Ensuring a robust supportive care with the use of dermoprotective agents will surely enhance faster recovery of such patients.
References
- Borda LJ, Ross A, Villada G, Milikowski C. Acute mucocutaneous methotrexate toxicity with marked tissue eosinophilia. BMJ Case Rep 2018; 2018: bcr2017221489
- Gupta A, Sardana K, Bhardwaj M, Singh A. Methotrexate cutaneous toxicity following a single dose of 10 mg in a case of chronic plaque psoriasis: a possible idiosyncratic reaction. Indian Dermatol Online J 2018; 9 (05) 328-330
- Knoll K, Anzengruber F, Cozzio A, French LE, Murer C, Navarini AA. Mucocutaneous ulcerations and pancytopenia due to methotrexate overdose. Case Rep Dermatol 2016; 8 (03) 287-293
- Karol SE, Yang W, Smith C. et al. Palmar-plantar erythrodysesthesia syndrome following treatment with high-dose methotrexate or high-dose cytarabine. Cancer 2017; 123 (18) 3602-3608
- Scheinfeld N. Three cases of toxic skin eruptions associated with methotrexate and a compilation of methotrexate-induced skin eruptions. Dermatol Online J 2006; 12 (07) 15
- Ramsey LB, Mizuno T, Vinks AA, O'Brien MM. Delayed methotrexate clearance in patients with acute lymphoblastic leukemia concurrently receiving dasatinib. Pediatr Blood Cancer 2019; 66 (05) e27618
- Schmiegelow
K. Advances in individual
prediction of methotrexate toxicity: a review. Br J Haematol 2009; 146 (05) 489-503
Address for correspondence
Anshul GuptaDepartment of Haematology, Sanjay Gandhi Postgraduate Institute of Medical SciencesRaebareli Road, Lucknow 226014, Uttar PradeshIndiaEmail: anshulhaemat@gmail.comPublication History
Article published online:
20 October 2022© 2022. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Figure 1:(A) Pruritic plaques on an erythematous base, (B) plantar erythrodysesthesia, (C) hyperkeratotic plaques over dorsum of hand, (D) oral mucositis and facial skin in healing stage with peeling of epidermis.
References
- Borda LJ, Ross A, Villada G, Milikowski C. Acute mucocutaneous methotrexate toxicity with marked tissue eosinophilia. BMJ Case Rep 2018; 2018: bcr2017221489
- Gupta A, Sardana K, Bhardwaj M, Singh A. Methotrexate cutaneous toxicity following a single dose of 10 mg in a case of chronic plaque psoriasis: a possible idiosyncratic reaction. Indian Dermatol Online J 2018; 9 (05) 328-330
- Knoll K, Anzengruber F, Cozzio A, French LE, Murer C, Navarini AA. Mucocutaneous ulcerations and pancytopenia due to methotrexate overdose. Case Rep Dermatol 2016; 8 (03) 287-293
- Karol SE, Yang W, Smith C. et al. Palmar-plantar erythrodysesthesia syndrome following treatment with high-dose methotrexate or high-dose cytarabine. Cancer 2017; 123 (18) 3602-3608
- Scheinfeld N. Three cases of toxic skin eruptions associated with methotrexate and a compilation of methotrexate-induced skin eruptions. Dermatol Online J 2006; 12 (07) 15
- Ramsey LB, Mizuno T, Vinks AA, O'Brien MM. Delayed methotrexate clearance in patients with acute lymphoblastic leukemia concurrently receiving dasatinib. Pediatr Blood Cancer 2019; 66 (05) e27618
- Schmiegelow K. Advances in individual prediction of methotrexate toxicity: a review. Br J Haematol 2009; 146 (05) 489-503


 PDF
PDF  Views
Views  Share
Share

