Imaging Recommendations for Diagnosis, Staging, and Management of Ovarian and Fallopian Tube Cancers
CC BY 4.0 · Indian J Med Paediatr Oncol 2023; 44(01): 100-109
DOI: DOI: 10.1055/s-0042-1759518
Abstract
Ovarian malignancy the third most common gynecological malignancy and is the leading cause of death in women. Non-specific clinical presentation delays the diagnosis, and they often present in the advanced stage of disease. No imaging modality is recommended for screening as there is no significant mortality reduction. Ultrasound (USG) is usually the initial modality in suspected ovarian mass. MRI is recommended for the characterization of indeterminate ovarian or adnexal mass on USG. CT abdomen and pelvis with oral and IV contrast is the recommended imaging modality in staging the disease, predicting the resectability and in selecting the patients who would benefit from neoadjuvant chemotherapy. Early ovarian cancers are staged by post-surgical histology and undergo upfront surgery. Advanced disease benefit by neoadjuvant chemotherapy and less morbidity by interval cytoreduction where image-guided biopsy is performed for histological diagnosis. Follow-up recommendations are based on tumor histology. CT/PET CT is recommended for diagnosing recurrence.
Publication History
Article published online:
24 January 2023
© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Ovarian malignancy the third most common gynecological malignancy and is the leading cause of death in women. Non-specific clinical presentation delays the diagnosis, and they often present in the advanced stage of disease. No imaging modality is recommended for screening as there is no significant mortality reduction. Ultrasound (USG) is usually the initial modality in suspected ovarian mass. MRI is recommended for the characterization of indeterminate ovarian or adnexal mass on USG. CT abdomen and pelvis with oral and IV contrast is the recommended imaging modality in staging the disease, predicting the resectability and in selecting the patients who would benefit from neoadjuvant chemotherapy. Early ovarian cancers are staged by post-surgical histology and undergo upfront surgery. Advanced disease benefit by neoadjuvant chemotherapy and less morbidity by interval cytoreduction where image-guided biopsy is performed for histological diagnosis. Follow-up recommendations are based on tumor histology. CT/PET CT is recommended for diagnosing recurrence.
Introduction
Ovarian cancer is the second most common gynecological cancer worldwide and third most common in developed countries. About 95%-of ovarian malignancies are epithelial origin and the rest arise from other subtypes. The two broad subtypes of epithelial ovarian cancers are type 1 that are low-grade tumors and type 2 that are aggressive tumors and most often present in advanced stages.[1] Though ovarian cancers are traditionally staged surgically, up to 40%-of patients may be under staged at laparotomy.[2] The role of the imaging is to characterize the ovarian lesion, determine the extent, predict primary resectability or unresectability, to evaluate the response to chemotherapy and localize the recurrence.[3]
Risk Factors and Etiopathogenesis
Several theories have been postulated about the origin of ovarian cancers. According to the World Health Organization (WHO), epithelial ovarian cancers are classified into high-grade serous and low-grade serous, mucinous, endometrioid, clear cell carcinomas and malignant Brenner tumor and carcinosarcomas.[4]
High-grade serous carcinomas are postulated to arise from the fimbrial end of the fallopian tube through precursor lesions called STIC (serous tubal intraepithelial carcinoma). Low-grade serous tumors may arise from benign or borderline tumors of the ovary.
Risk factors for ovarian cancer include genetic mutations such as BRCA 1 and 2 and Lynch syndrome, nulliparity, endometriosis, obesity, and smoking.
Protective factors include use of oral contraceptive pills, breastfeeding, tubectomy, and tubal ligation.[5]
Epidemiology in India and Globally
Ovarian cancer currently ranks as the seventh most common cancer in women, worldwide. Often called a “silent killer,” it has high mortality rates due to its insidious onset and lack of specific symptoms. It occurs more commonly in developed countries such as the US and Europe.[6] However, mortality rates are highest in Asian and African countries with the existing disparities in access to healthcare and affordability. In Asia, the highest ovarian cancer-related mortality rate is seen in India.[7] Carcinoma ovary is the third most common cancer in Indian women followed by breast and cervix. The cumulative risk of developing ovarian cancer between 0 and 74 years of age is about 1 in 133.[8]
Imaging Referral Guidelines
No imaging is recommended for screening for ovarian carcinoma in the general population as there was no significant reduction in mortality rates due to screening according to ovarian cancer population screening and mortality after long-term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) trial.[9] Ultrasonogram (USG) with CA-125 levels may be considered for women with hereditary cancer syndromes starting at 30 to 35 years of age if they have not undergone risk-reducing salpingo-oophorectomy.[10]
USG is the initial imaging modality of choice in suspected ovarian lesions. If the lesion is suspected to be malignant on USG, staging CT abdomen and pelvis with oral and IV contrast is indicated to evaluate the extent of disease and identify patients who would benefit from neoadjuvant chemotherapy.[11] [12] If the lesion is indeterminate on USG, MRI is recommended for further evaluation.[13]
FDG- PET-CT is helpful for patients with primary peritoneal carcinomatosis or elevated tumor markers with inconclusive CT findings.[14]
Serum CA-125 levels and CT are the standard tools for assessing the response to chemotherapy.[15] FDG-PET-CT is useful in early prediction of response after neoadjuvant chemotherapy due to its functional imaging.[16] In MR imaging, DWI is useful in early prediction of response by an increase in ADC values.[17]
Rising CA-125 levels, symptoms and signs of relapse after treatment prompts imaging evaluation for recurrence. CT chest, abdomen, and pelvis is the imaging modality of choice in clinically suspected/known recurrence of disease in carcinoma ovary.[14] PET-CT has similar or higher accuracy compared to CT in detecting recurrences.[18]
Clinical and Diagnostic Work-up Excluding Imaging
Women with ovarian malignancies usually have a wide range of symptoms from vague abdominal discomfort, painless abdominal distension, mass per abdomen and bowel or bladder disturbances. Rarer clinical symptoms include acute abdomen secondary to ovarian torsion, bowel obstruction, gastrointestinal bleeds, vaginal bleeds, lymphadenopathy, and paraneoplastic syndromes.
The serum tumor markers remain the easiest and sensitive screening tool, albeit non-specific. Serum levels of cancer antigen 125 (CA 125) show sensitivity of 78%-and specificity of 77%-for epithelial tumors. Higher sensitivity and specificity are observed in postmenopausal women, advanced stage, and higher grade.[19] [20] The human epididymis protein 4 (HE 4) level may be useful in patients with low/normal CA 125. CEA and CA 19.9 are other markers that can also be elevated.
Specific tumor markers such as inhibin B alfa feto protein (AFP) and beta human chorionic gonadotropin (beta HCG) lactate dehydrogenase (LDH) are used for diagnosis and follow-up of non-epithelial tumors.
Biopsy of ovarian masses is generally not recommended as rupture and peritoneal seeding can upstage the tumor. In patients with ascites, diagnostic cytology yields vary in 30 to 70%. In women who are not eligible for surgical cytoreduction, image-guided biopsies or laparoscopic biopsy may establish diagnosis.[21] [22] [23]
Additional evaluation with upper GI endoscopy and colonoscopy should be considered for all women with clinic-radiological suspicion or elevated serum markers suggestive of gastrointestinal primary.
Irrespective of family history, these patients should be offered genetic testing that has an impact on treatment plan and choice and after treatment care.[24] Patients with epithelial carcinoma of the ovary should be offered testing for BRCA1 or BRCA2 mutations and for Lynch syndrome. Patients with mucinous, clear cell, endometrioid cancers are offered testing for DNA mismatch repair deficiency.
Imaging Guidelines
Screening for Ovarian Cancer
Ovarian cancer is the most common cause of cancer deaths due to gynecological malignancies. Because it presents with non-specific symptoms, it is diagnosed in the advanced stage in 58%-of patients which results in a low 5-year survival rate (30%). When diagnosed early as a localized disease, the 5-year survival rate is 93%.[25] This led to the development of screening tools for ovarian cancer.
The common screening tools considered are transvaginal USG and serum CA-125 levels. Both have the disadvantage of high false-positive rates leading to unnecessary interventions. According to the randomised controlled trials on ovarian cancer screening, there was no significant reduction in mortality rates due to ovarian cancer with screening.[9] [26] , Because of the negative net benefit and risk ratio, it is not recommended to screen asymptomatic high risk women.[27] [28] [29]
For high risk women, risk-reducing salpingo-oophorectomy(RRSO) is recommended at 35 to 40 years of age and upon completion of child bearing. High-risk women who have not elected RRSO, screening with transvaginal sonography and CA-125 levels, although of uncertain benefit, is recommended at the clinician's discretion starting at the age of 30 to 35 years.[8]
Diagnosis
The initial imaging modality of choice is ultrasonography in a suspected adnexal or ovarian mass (USG).[10] The International Ovarian Tumour Analysis (IOTA) and Ovarian-Adnexal Reporting and Data System (O-RADS) may be used for the characterization and risk stratification of adnexal masses.[30] If the lesion is benign, it can be followed up or no further evaluation is recommended.
If the USG findings are indicative of a lesion with high risk of malignancy, evaluation by a gyneco-oncologist along with CT of the abdomen and pelvis or CT thorax, abdomen and pelvis is recommended for staging of the disease and treatment planning.[11] In patients with indeterminate features, MRI of the abdomen and pelvis is recommended for further characterization.[11]
In the evaluation of indeterminate adnexal lesions, MRI is a superior modality than USG/CT. MRI has increased specificity compared with the USG, decreasing the number of false-positive diagnoses for malignancy and thereby avoiding unnecessary or over-extensive surgery.[31]
The Ovarian-Adnexal Reporting and Data System (O-RADS) is released by American College of Radiology for USG and MRI.[30] [31] It assigns a probability of malignancy based on the imaging features of an adnexal lesion and provides information to facilitate optimal patient management and a uniform reporting system with standardized lexicons. The primary goal of the O-RADS risk stratification system is to improve communication between radiologists and referring physicians in a reproducible fashion, so that women with benign lesions or borderline tumors can avoid unnecessary or over-extensive surgery, respectively, and women with potential malignancy are promptly referred for gynecologic oncologic surgical evaluation.
The classical benign adnexal lesions on ultrasound include unilocular cyst < 10 cm with smooth inner walls and also typical dermoid cysts, typical hemorrhagic and endometriosis cysts, hydrosalpinx and paraovarian/peritoneal inclusion cysts. On MRI, typical benign feature is cysts with T2 dark/DWI dark solid components.[30] [31]
High-risk features that indicates malignancy in adnexal masses on ultrasound include unilocular cyst with > 4 papillary projections, multilocular cyst with solid component with color score of 3 to 4, solid lesion with smooth outer contour with color score of 4, solid lesion with irregular outer contour and when the lesion is associated with ascites and/or peritoneal nodules. Solid tissue with high-risk time intensity curve in dynamic post contrast MRI is a high-risk feature.[30] [31]
Indeterminate features of adnexal masses on ultrasound include unilocular cyst > 10 cm in size or with irregular inner walls, multilocular cyst, multilocular cyst with solid component with a color score of 1 to 2. Unilocular cyst with 1 to 3 papillary projections or solid components and solid lesion with smooth outer contour with color score of 1 to 3. High-risk features on MRI include solid components showing low-risk or intermediate-risk time intensity curve on dynamic post contrast images.[30] [31]
Image-Guided Intervention for Diagnosis
In ovarian cancer patients amenable to primary cytoreductive surgery, definitive diagnosis is by surgical histopathology.
Image-guided biopsy is recommended only in patients who are not amenable for primary cytoreductive surgery. Trans-abdominal or trans-vaginal ultrasound-guided biopsy of omental, peritoneal, or adnexal mass can be done to confirm diagnosis and histopathological type of ovarian cancer before starting optimal neoadjuvant chemotherapy. If biopsy is not feasible, ascitic or pleural fluid aspiration, cystoscopy, and CA-125:CEA ratio of > 25 can be used for diagnosis.[3] In patients with pleural effusion, nature of effusion must be confirmed with pleural fluid aspiration and cytology.
Staging
Contrast-enhanced CT (CECT) of the abdomen and pelvis or CECT thorax, abdomen and pelvis is the recommended imaging for staging of ovarian cancer. CT plays an important role in the assessment of operability and for identifying lesions in regions that are difficult to resect
CT characterizes the tubo-ovarian lesion and detects the involvement of the adjacent pelvic organs such as infiltration into the uterus, rectum, and sigmoid colon, involvement of the ureter. It also detects the extension of the disease outside the pelvis with involvement of the peritoneum, omentum, mesentery, visceral organs, and lymph nodes ([Fig.1]). The reported accuracy of CT in staging of ovarian cancer is up to 94%.[14]
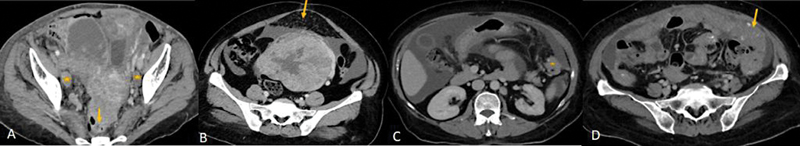
| Figure 1:(A) CECT of a patient with ovarian carcinoma: Lesion with irregular solid tissue in right adnexa and irregular solid lesion in left adnexa with infiltration into the anterior wall of the rectum (yellow arrow) and uterus and necrotic bilateral external iliac lymph-nodes (*). (B-D) Spectrum of omental involvement in advanced ovarian carcinoma along with massive ascites. (B) omental fat stranding and nodularity (yellow arrow), (C) omental deposit (*), (D)- omental caking(arrow).
Further CT detects the involvement of certain sites such as the mesenteric root, gastrosplenic ligament, lesser sac, porta hepatis, hepatic intersegmental fissures, subdiaphragmatic regions, infiltrating liver, and splenic deposits and also helps in detecting lymphadenopathy at or above the celiac axis, extraperitoneal disease, and pelvic sidewall invasion and thereby predicts non resectability ([Fig. 2]).[14] The limitation of the CT is to detect deposits that are less than 5 mm within the peritoneum, bowel surface especially when there is no ascites.[32] For deposits that are < 5 mm, the sensitivity of CT is only 11%.[33] Positive oral and rectal contrast improves detection of visceral peritoneal deposits. CT chest can be used in cases of suspected pleural or pulmonary metastasis.
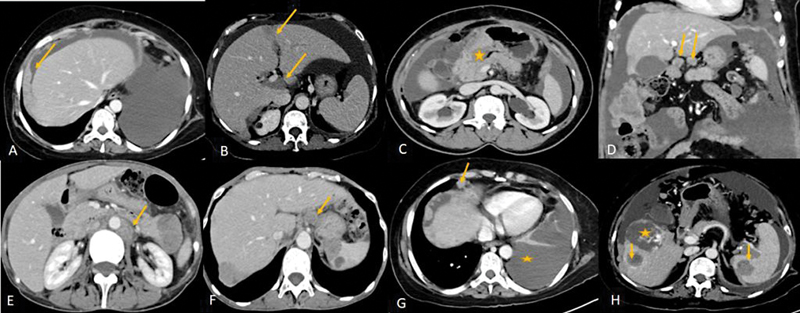
| Figure 2:(A-E) CECT showing some of the unfavorable sites of involvement which makes complete cytoreduction less likely. (A) Plaque like subdiaphragmatic disease(arrow), (B) intersegmental fissures of liver and porta (arrows), (C) Disease encasing the stomach (*), (D) Lesser omentum (arrows), (E)lymph-nodes above the level of renal hilum(arrow). (F-H: Metastatic disease in carcinoma ovary: F: Celiac lymph-node (arrow). (G) Anterior cardiophrenic lymph-node (arrow) and malignant left pleural effusion (*) (H) Liver and splenic intraparenchymal deposits(arrows), * - incidental hemangioma in liver
Alternatively, MRI and FDG-PET-CT may be appropriate for staging. MRI has equivalent accuracy to CT in staging of ovarian cancer with sensitivity of 0.88, specificity of 0.74, and accuracy of 0.84.[34] However, the limitations are that MRI is more sensitive to motion and has long duration of study compared to CT. FDG PET has a specificity as low as 54%-and sensitivity of 86%-in diagnosis and treatment of ovarian cancers. PET CT can be false positive in certain benign tumors such as fibroma and dermoid and in non-neoplastic conditions such as hydrosalpinx and endometriosis. However, various studies demonstrate that when combined with CT, it has a higher accuracy than FDG-PET or CT alone.[35] [36] Others imaging modalities such as non-contrast CT, ultrasound of abdomen and pelvis are not recommended for staging.[14]
CT is also recommended to assess response in patients who are undergoing neoadjuvant chemotherapy before interval debulking ([Fig. 3]).[14]
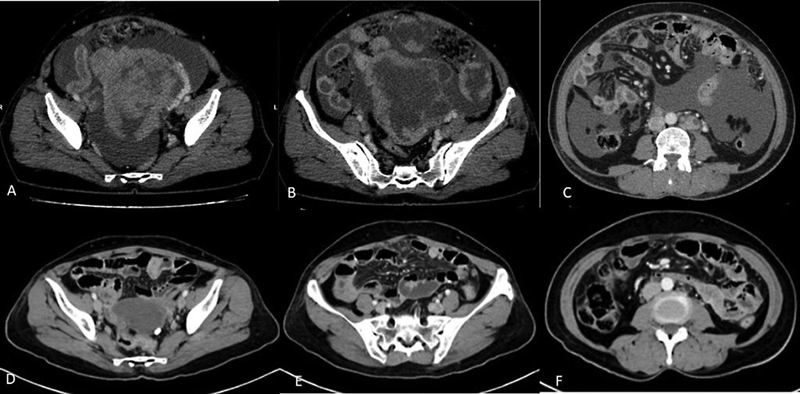
| Figure 3:(A-F) Role of contrast-enhanced CT in assessing the response after neoadjuvant chemotherapy. CECT images before (A-C) and after (D-F) 3 cycles of neoadjuvant chemotherapy show significant reduction in size of the primary mass lesion in the pelvis (A and D) and omental deposits (B and E) and resolution of ascites and retroperitoneal lymph-nodes (C and F).
The 2014 revised FIGO staging classification is used for staging of ovarian, fallopian tube, and peritoneal malignancies.[37] In the recent FIGO 2021 staging report, there are no changes in the staging system. FIGO classification along with equivalent stages in the Union of International Cancer Control (UICC) TNM staging is given in [Table 1].
|
UICC stage |
FIGO Stage |
Stage description |
|---|---|---|
|
T1N0M0 |
I |
The tumor is limited to the ovary (or ovaries) or fallopian tube(s). |
|
T1aN0M0 |
IA |
The tumor is limited to one ovary with an intact capsule or one fallopian tube. There is no tumor on the surface of the ovary or fallopian tube. No cancer cells are found in the ascitic fluid or peritoneal washings. |
|
T1bN0M0 |
IB |
The tumor is limited to both ovaries or fallopian tubes but not on their outer surfaces. No cancer cells are found in the ascitic fluid or peritoneal washings. |
|
T1cN0M0 |
IC |
The cancer is in one or both ovaries or fallopian tubes and any of the following are present: IC1: rupture and spillage of tumor during surgery IC2: capsule rupture before surgery or tumor on ovarian or fallopian tube surface IC3: tumor cells in the ascites or peritoneal washings |
|
T2N0M0 |
II |
Involvement of 1 or both ovaries or fallopian tubes with extension to pelvis (below pelvic brim) or primary peritoneal cancer. |
|
T2aN0M0 |
IIA |
Extension/implants on the uterus and/or the fallopian tubes and/ or the ovaries. |
|
T2bN0M0 |
IIB |
Involvement of other intraperitoneal pelvic structures |
|
T1-3N0-1M0 |
III |
Involvement of 1 or both ovaries or fallopian tubes, or peritoneal cancer with spread to the peritoneum outside the pelvis confirmed by cytology or histology and/or metastasis to the retroperitoneal lymph nodes |
|
T1-2 N1M0 |
IIIA1 |
Positive retroperitoneal lymph nodes (cytologically or histologically proven) IIIA1(i) Metastasis up to 10 mm in greatest dimension IIIA1(ii) Metastasis more than 10 mm in greatest dimension |
|
T3a2N0-1M0 |
IIIA2 |
Microscopic involvement of extra pelvic peritoneum with or without positive retroperitoneal lymph nodes |
|
T3bN0-1M0 |
IIIB |
Macroscopic deposits in the extra pelvic peritoneum, with largest deposit less than 2 cm in size with or without retroperitoneal lymph nodes |
|
T3cN0-1M0 |
IIIC |
Macroscopic deposits in the extra pelvic peritoneum with largest deposit more than 2 cm in size (includes extension of tumor to capsule of the liver and spleen without parenchymal involvement of either organ) |
|
Any T Any N M1 |
IVA |
Pleural effusion with positive cytology |
|
Any T Any N M1 |
IVB |
Parenchymal metastases to solid organs and metastases to extra-abdominal organs (including inguinal lymph nodes and lymph nodes outside of the abdominal cavity) |
|
Histological type |
Follow-up recommendation |
Indications for imaging |
|---|---|---|
|
Epithelial high-grade serous carcinoma of ovary, fallopian tube, and peritoneum |
Once in 3 months in first year Once in 4–6 months until 5 years Annually after 5 years |
Not routinely indicated. Indicated only if there are 1. symptoms and/or signs of recurrence 2. Rising CA-125 levels 3. when tumor markers or physical exam is unreliable |
|
Low-grade serous cancers |
Similar as high-grade tumors but at less frequent intervals |
Same as above |
|
Borderline tumors |
Similar as high-grade tumors but at less frequent intervals |
Same as above + transvaginal sonography if one ovary is preserved |
|
Mucinous tumors |
Similar as high-grade serous tumors |
Similar as high-grade serous tumors |
|
Granulosa cell tumors |
Once in 6–12 months if early stage, low risk Once in 4–6 months if high risk |
Reserved for patients with symptoms and signs or elevated biomarkers |
|
Dysgerminoma |
Year 1-Every 2–3 months Year 2-Every 3–4 months Year 3-Every 6 months Year 4–5-Every 6 months After 5 years-annually |
Year 1-abdominal/pelvic CT (every 3–4 months) Year 2-abdominal/pelvic CT (every 6 months) Year 3-abdominal/pelvic CT (annually) Year 4–5-abdominal/pelvic CT (annually) After 5 years-as clinically indicated |
|
Non-dysgerminoma |
Year 1-Every 2 months Year 2-Every 2 months Year 3-Every 4–6 months Year 4–5-Every 6 months After 5 years-annually |
Year 1-Chest/abdominal/pelvic CT (every 3–4 months) Year 2-Chest/abdominal/pelvic CT (every 4–6 months) Year 3-Abdominal/pelvic CT (every 6–12 months) Year 4–5-Abdominal/pelvic CT (every 6–12 months) After 5 years-As clinically indicated |

| Figure 4:(A, B) CECT and FDG PET-CT showing recurrence along the wall of the rectum in a patient with carcinoma ovary post neoadjuvant chemotherapy, interval debulking followed by adjuvant chemotherapy with elevated CA-125 levels on follow up.
Principles of Management
Depending on the stage and extent of disease, ovarian cancer patients are managed with primary cytoreduction (removal of uterus, tubes and ovaries, omentum, peritoneal biopsy and lymph-node dissection), secondary cyto-reduction following neo-adjuvant chemotherapy, palliative intent chemotherapy, and best supportive care.
Chemotherapy may be omitted in low-grade, stage IA or IB cancers. All other stages are given four to six cycles of adjuvant chemotherapy. Fertility-sparing surgery may be an option in ovary-confined disease, if the woman wishes to consider future child-bearing options.
The principle of management in advanced stages is “optimal debulking (removal of all macroscopic disease)” followed by adjuvant chemotherapy.[43] [44]
The preferred chemotherapy regimen is six cycles of paclitaxel and carboplatin. Neoadjuvant chemotherapy with interval cytoreductive surgery has become a preferred option, especially in high-volume disease.[44] [45] [46]
Targeted therapies such as bevacizumab and PARP inhibitors, have shown to improve overall or progression survival, especially in women with genetic mutations such as BRCA 1 and 2.[47] [48] Hyper-thermic intraperitoneal chemotherapy (HIPEC) is also given in optimally debulked advanced stage III/IV ovarian cancers. [Table 3] summarizes the principles of management.
|
Primary treatment |
Early stage (Stages I and II) |
Surgery-staging laparotomy |
Stage IA and IB of low-grade serous, mucinous, and grade I-endometrioid, stage IA of clear cell histology |
Observation observation/chemotherapy |
|
All high-grade serous, grade 2,3-endometrioid stage IC and above of low-grade serous, mucinous and grade I-endometrioid stage IB and above of clear cell histology |
6# adjuvant chemotherapy |
|||
|
Advanced stage (stages III and IV) |
Surgery–primary cytoreduction |
6# adjuvant chemotherapy ± targeted therapy |
||
|
3# NACT in select stage IIIC/IV |
Surgery–interval cytoreduction |
3# adjuvant CT ± targeted therapy |
||
|
Treatment of relapsed disease |
Platinum-refractory/resistant relapse (no response progression during or within 6 months of completion of platinum based chemotherapy) |
Single-agent chemotherapy/best supportive care [paclitaxel, gemcitabine, topotecan, pegylated liposomal doxorubicin (PLD)] |
||
|
Platinum-sensitive relapse (progression more than 6 months after completion of previous platinum chemotherapy) |
Platinum based combination chemotherapy ± targeted therapy |
|||
|
Isolated serological relapse (elevation of CA-125 levels alone) |
Can be observed until symptomatic/radiological evidence of relapse (decision to be individualized) |
|||
|
Long disease-free interval and localized relapse |
Surgical resection of relapsed disease may be considered |
|||
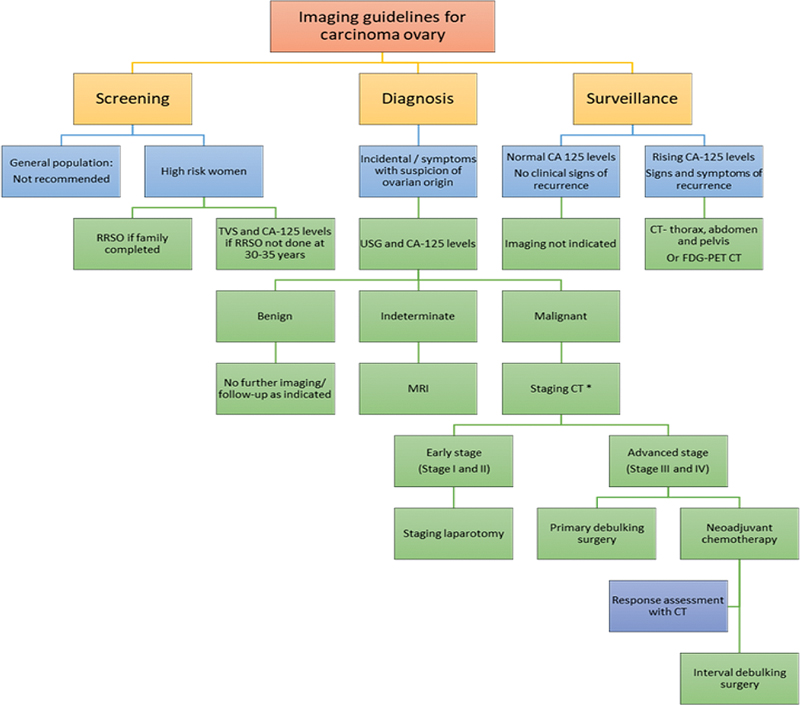
| Figure 5:Imaging guidelines for carcinoma ovary. RRSO, risk reducing salpingo-oophorectomy; TVS, transvaginal sonography. *Apart from CT, other equivalent appropriate modalities for staging include, MRI abdomen and pelvis with contrast and FDG PET-CT.
Summary of Recommendations
No screening tests or imaging are recommended even for high-risk patients for detection of ovarian/tubal cancers.
Ovarian cancers are primarily staged through primary cytoreductive surgery and the pathology is confirmed surgical histopathology.
Staging through imaging is best done with CT of the abdomen and pelvis with oral and IV contrast and CT chest is a useful addition in those with pleural effusion.
CT abdomen and pelvis with oral and IV contrast is also used to assess the response to neoadjuvant chemotherapy before interval debulking surgery.
In case of suspected recurrence, contrast-enhanced CT thorax, abdomen, and pelvis with oral contrast is the imaging modality of choice. FDG PET CT may be considered when CT findings are inconclusive and there is high clinical suspicion of recurrence.
Conflict of Interest
None declared.
References
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65 (02) 87-108
- Forstner R, Hricak H, Occhipinti KA, Powell CB, Frankel SD, Stern JL. Ovarian cancer: staging with CT and MR imaging. Radiology 1995; 197 (03) 619-626
- NCCN Clinical Practice Guidelines in Oncology. Epithelial ovarian cancer (including fallopian tube cancer and primary peritoneal cancer). Version 1.2022. Accessed November 09, 2022, at: https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf
- Duska LR, Kohn EC. The new classifications of ovarian, fallopian tube, and primary peritoneal cancer and their clinical implications. Ann Oncol 2017; 28 (suppl_8): viii8-viii12 DOI: 10.1093/annonc/mdx445.
- Momenimovahed Z, Tiznobaik A, Taheri S, Salehiniya H. Ovarian cancer in the world: epidemiology and risk factors. Int J Womens Health 2019; 11: 287-299 DOI: 10.2147/IJWH.S197604.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68 (06) 394-424 DOI: 10.3322/caac.21492.
- Malvezzi M, Carioli G, Rodriguez T, Negri E, La Vecchia C. Global trends and predictions in ovarian cancer mortality. Ann Oncol 2016; 27 (11) 2017-2025 DOI: 10.1093/annonc/mdw306.
- Mathur P, Sathishkumar K, Chaturvedi M. et al; ICMR-NCDIR-NCRP Investigator Group. Cancer Statistics, 2020: Report From National Cancer Registry Programme, India. JCO Glob Oncol 2020; 6 (06) 1063-1075 DOI: 10.1200/GO.20.00122.
- Menon U, Gentry-Maharaj A, Burnell M. et al. Ovarian cancer population screening and mortality after long-term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet 2021; 397 (10290): 2182-2193 DOI: 10.1016/S0140-6736(21)00731-5.
- Daly MB, Pal T, Berry MP. et al; Genetic/familial high-risk assessment: breast, ovarian, and pancreatic, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2021; 19 (01) 77-102 DOI: 10.6004/jnccn.2021.0001.
- Atri M, Alabousi A, Reinhold C. et al; Expert Panel on Women's Imaging. ACR appropriateness criteria® clinically suspected adnexal mass, no acute symptoms. J Am Coll Radiol 2019; 16 (5S): S77-S93 DOI: 10.1016/j.jacr.2019.02.011.
- Schmidt S, Meuli RA, Achtari C, Prior JO. Peritoneal carcinomatosis in primary ovarian cancer staging: comparison between MDCT, MRI, and 18F-FDG PET/CT. Clin Nucl Med 2015; 40 (05) 371-377
- Liu J, Xu Y, Wang J. Ultrasonography, computed tomography and magnetic resonance imaging for diagnosis of ovarian carcinoma. Eur J Radiol 2007; 62 (03) 328-334
- Kang SK, Reinhold C, Atri M. et al; Expert Panel on Women's Imaging. ACR appropriateness criteria® staging and follow-up of ovarian cancer. J Am Coll Radiol 2018; 15 (5S): S198-S207 DOI: 10.1016/j.jacr.2018.03.015.
- Rockall A, Munari A, Avril N. New ways of assessing ovarian cancer response: metabolic imaging and beyond. Cancer Imaging 2012; 12 (02) 310-314 DOI: 10.1102/1470-7330.2012.9004.
- Vallius T, Hynninen J, Kemppainen J. et al. 18F-FDG-PET/CT based total metabolic tumor volume change during neoadjuvant chemotherapy predicts outcome in advanced epithelial ovarian cancer. Eur J Nucl Med Mol Imaging 2018; 45 (07) 1224-1232 DOI: 10.1007/s00259-018-3961-z.
- Winfield JM, Wakefield JC, Dolling D. et al. Diffusion-weighted MRI in advanced epithelial ovarian cancer: apparent diffusion coefficient as a response marker. Radiology 2019; 293 (02) 374-383 DOI: 10.1148/radiol.2019190545.
- Sebastian S, Lee SI, Horowitz NS. et al. PET-CT vs. CT alone in ovarian cancer recurrence. Abdom Imaging 2008; 33 (01) 112-118 DOI: 10.1007/s00261-007-9218-0.
- Bast Jr RC, Klug TL, St John E. et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med 1983; 309 (15) 883-887
- Dodge JE, Covens AL, Lacchetti C. et al; Gynecology Cancer Disease Site Group. Preoperative identification of a suspicious adnexal mass: a systematic review and meta-analysis. Gynecol Oncol 2012; 126 (01) 157-166
- Hewitt MJ, Anderson K, Hall GD. et al. Women with peritoneal carcinomatosis of unknown origin: Efficacy of image-guided biopsy to determine site-specific diagnosis. BJOG 2007; 114 (01) 46-50
- Fagotti A, Ferrandina G, Fanfani F. et al. A laparoscopy-based score to predict surgical outcome in patients with advanced ovarian carcinoma: a pilot study. Ann Surg Oncol 2006; 13 (08) 1156-1161
- Rutten MJ, van Meurs HS, van de Vrie R. et al. Laparoscopy to predict the result of primary cytoreductive surgery in patients with advanced ovarian cancer: a randomized controlled trial. J Clin Oncol 2017; 35 (06) 613-621
- Konstantinopoulos PA, Norquist B, Lacchetti C. et al. Germline and somatic tumor testing in epithelial ovarian cancer: ASCO guideline. J Clin Oncol 2020; 38 (11) 1222-1245
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin 2021; 71 (01) 7-33
- Buys SS, Partridge E, Black A. et al; PLCO Project Team. Effect of screening on ovarian cancer mortality: the prostate, lung, colorectal and ovarian (PLCO) cancer screening randomized controlled trial. JAMA 2011; 305 (22) 2295-2303 DOI: 10.1001/jama.2011.766.
- Grossman DC, Curry SJ, Owens DK. et al; US Preventive Services Task Force. Screening for ovarian cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018; 319 (06) 588-594 DOI: 10.1001/jama.2017.21926.
- Committee on Gynecologic Practice, Society of Gynecologic Oncology. Committee Opinion No. 716: the role of the obstetrician-gynaecologist in the early detection of epithelial ovarian cancer in women at average risk. Obstet Gynecol 2017; 130 (03) e146-e149
- Smith RA, Andrews KS, Brooks D. et al. Cancer screening in the United States, 2017: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin 2017; 67 (02) 100-121 DOI: 10.3322/caac.21392.
- Andreotti RF, Timmerman D, Strachowski LM. et al. O-RADS US risk stratification and management system: a consensus guideline from the ACR Ovarian-Adnexal Reporting and Data System Committee. Radiology 2020; 294 (01) 168-185 DOI: 10.1148/radiol.2019191150.
- Sadowski EA, Thomassin-Naggara I, Rockall A. et al. O-RADS MRI risk stratification system: guide for assessing adnexal lesions from the ACR O-RADS Committee. Radiology 2022; 303 (01) 35-47 DOI: 10.1148/radiol.204371.
- Hynninen J, Kemppainen J, Lavonius M. et al. A prospective comparison of integrated FDG-PET/contrast-enhanced CT and contrast-enhanced CT for pretreatment imaging of advanced epithelial ovarian cancer. Gynecol Oncol 2013; 131 (02) 389-394
- Koh JL, Yan TD, Glenn D, Morris DL. Evaluation of preoperative computed tomography in estimating peritoneal cancer index in colorectal peritoneal carcinomatosis. Ann Surg Oncol 2009; 16 (02) 327-333 DOI: 10.1245/s10434-008-0234-2.
- Low RN, Barone RM. Combined diffusion-weighted and gadolinium-enhanced MRI can accurately predict the peritoneal cancer index preoperatively in patients being considered for cytoreductive surgical procedures. Ann Surg Oncol 2012; 19 (05) 1394-1401
- Castellucci P, Perrone AM, Picchio M. et al. Diagnostic accuracy of 18F-FDG PET/CT in characterizing ovarian lesions and staging ovarian cancer: correlation with transvaginal ultrasonography, computed tomography, and histology. Nucl Med Commun 2007; 28 (08) 589-595 DOI: 10.1097/MNM.0b013e3281afa256.
- Nam EJ, Yun MJ, Oh YT. et al. Diagnosis and staging of primary ovarian cancer: correlation between PET/CT, Doppler US, and CT or MRI. Gynecol Oncol 2010; 116 (03) 389-394
- Mutch DG, Prat J. 2014 FIGO staging for ovarian, fallopian tube and peritoneal cancer. Gynecol Oncol 2014; 133 (03) 401-404
- Berek JS, Renz M, Kehoe S, Kumar L, Friedlander M. Cancer of the ovary, fallopian tube, and peritoneum: 2021 update. Int J Gynaecol Obstet 2021; 155 (Suppl 1): 61-85 DOI: 10.1002/ijgo.13878.
- Tawakol A, Abdelhafez YG, Osama A, Hamada E, El Refaei S. Diagnostic performance of 18F-FDG PET/contrast-enhanced CT versus contrast-enhanced CT alone for post-treatment detection of ovarian malignancy. Nucl Med Commun 2016; 37 (05) 453-460
- Gu P, Pan LL, Wu SQ, Sun L, Huang G. CA 125, PET alone, PET-CT, CT and MRI in diagnosing recurrent ovarian carcinoma: a systematic review and meta-analysis. Eur J Radiol 2009; 71 (01) 164-174
- Nakamoto Y, Saga T, Ishimori T. et al. Clinical value of positron emission tomography with FDG for recurrent ovarian cancer. Am J Roentgenol 2001; 176 (06) 1449-1454
- Salani R, Backes FJ, Fung MFK. et al. Posttreatment surveillance and diagnosis of recurrence in women with gynecologic malignancies: Society of Gynecologic Oncologists recommendations. Am J Obstet Gynecol 2011; 204 (06) 466-478
- Chi DS, Eisenhauer EL, Zivanovic O. et al. Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Gynecol Oncol 2009; 114 (01) 26-31 DOI: 10.1016/j.ygyno.2009.03.018.
- Elattar A, Bryant A, Winter-Roach BA, Hatem M, Naik R. Optimal primary surgical treatment for advanced epithelial ovarian cancer. Cochrane Database Syst Rev 2011; (08) CD007565 DOI: 10.1002/14651858.CD007565.pub2.
- Kehoe S, Hook J, Nankivell M. et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet 2015; 386 (9990): 249-257 DOI: 10.1016/S0140-6736(14)62223-6.
- Makar AP, Tropé CG, Tummers P, Denys H, Vandecasteele K. Advanced ovarian cancer: primary or interval debulking? Five categories of patients in view of the results of randomized trials and tumor biology: primary debulking surgery and interval debulking surgery for advanced ovarian cancer. Oncologist 2016; 21 (06) 745-754 DOI: 10.1634/theoncologist.2015-0239.
- Tewari KS, Burger RA, Enserro D. et al. Final overall survival of a randomized trial of bevacizumab for primary treatment of ovarian cancer. J Clin Oncol 2019; 37 (26) 2317-2328 DOI: 10.1200/JCO.19.01009.
- Moore K, Colombo N, Scambia G. et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 2018; 379 (26) 2495-2505 DOI: 10.1056/NEJMoa1810858.
- Rustin GJS. Follow-up with CA125 after primary therapy of advanced ovarian cancer has major implications for treatment outcome and trial performances and should not be routinely performed. Ann Oncol 2011; 22 (Suppl 8) viii45-viii48 DOI: 10.1093/annonc/mdr471.
- Esselen KM, Cronin
AM, Bixel K. et al. Use of CA-125 tests
and
computed tomographic scans for surveillance in ovarian cancer. JAMA Oncol 2016; 2 (11) 1427-1433
DOI: 10.1001/jamaoncol.2016.1842.
Address for correspondence
Renganathan Rupa, DNB, FRCRDepartment of Diagnostic and Interventional Radiology, Division of Breast and Women's Imaging and Interventions, Kovai Medical Center and HospitalsCoimbatore, Tamil Nadu 641014IndiaEmail: drrrupa@gmail.comPublication History
Article published online:
24 January 2023© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Figure 1:(A) CECT of a patient with ovarian carcinoma: Lesion with irregular solid tissue in right adnexa and irregular solid lesion in left adnexa with infiltration into the anterior wall of the rectum (yellow arrow) and uterus and necrotic bilateral external iliac lymph-nodes (*). (B-D) Spectrum of omental involvement in advanced ovarian carcinoma along with massive ascites. (B) omental fat stranding and nodularity (yellow arrow), (C) omental deposit (*), (D)- omental caking(arrow).

| Figure 2:(A-E) CECT showing some of the unfavorable sites of involvement which makes complete cytoreduction less likely. (A) Plaque like subdiaphragmatic disease(arrow), (B) intersegmental fissures of liver and porta (arrows), (C) Disease encasing the stomach (*), (D) Lesser omentum (arrows), (E)lymph-nodes above the level of renal hilum(arrow). (F-H: Metastatic disease in carcinoma ovary: F: Celiac lymph-node (arrow). (G) Anterior cardiophrenic lymph-node (arrow) and malignant left pleural effusion (*) (H) Liver and splenic intraparenchymal deposits(arrows), * - incidental hemangioma in liver

| Figure 3:(A-F) Role of contrast-enhanced CT in assessing the response after neoadjuvant chemotherapy. CECT images before (A-C) and after (D-F) 3 cycles of neoadjuvant chemotherapy show significant reduction in size of the primary mass lesion in the pelvis (A and D) and omental deposits (B and E) and resolution of ascites and retroperitoneal lymph-nodes (C and F).

| Figure 4:(A, B) CECT and FDG PET-CT showing recurrence along the wall of the rectum in a patient with carcinoma ovary post neoadjuvant chemotherapy, interval debulking followed by adjuvant chemotherapy with elevated CA-125 levels on follow up.

| Figure 5:Imaging guidelines for carcinoma ovary. RRSO, risk reducing salpingo-oophorectomy; TVS, transvaginal sonography. *Apart from CT, other equivalent appropriate modalities for staging include, MRI abdomen and pelvis with contrast and FDG PET-CT.
References
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65 (02) 87-108
- Forstner R, Hricak H, Occhipinti KA, Powell CB, Frankel SD, Stern JL. Ovarian cancer: staging with CT and MR imaging. Radiology 1995; 197 (03) 619-626
- NCCN Clinical Practice Guidelines in Oncology. Epithelial ovarian cancer (including fallopian tube cancer and primary peritoneal cancer). Version 1.2022. Accessed November 09, 2022, at: https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf
- Duska LR, Kohn EC. The new classifications of ovarian, fallopian tube, and primary peritoneal cancer and their clinical implications. Ann Oncol 2017; 28 (suppl_8): viii8-viii12 DOI: 10.1093/annonc/mdx445.
- Momenimovahed Z, Tiznobaik A, Taheri S, Salehiniya H. Ovarian cancer in the world: epidemiology and risk factors. Int J Womens Health 2019; 11: 287-299 DOI: 10.2147/IJWH.S197604.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68 (06) 394-424 DOI: 10.3322/caac.21492.
- Malvezzi M, Carioli G, Rodriguez T, Negri E, La Vecchia C. Global trends and predictions in ovarian cancer mortality. Ann Oncol 2016; 27 (11) 2017-2025 DOI: 10.1093/annonc/mdw306.
- Mathur P, Sathishkumar K, Chaturvedi M. et al; ICMR-NCDIR-NCRP Investigator Group. Cancer Statistics, 2020: Report From National Cancer Registry Programme, India. JCO Glob Oncol 2020; 6 (06) 1063-1075 DOI: 10.1200/GO.20.00122.
- Menon U, Gentry-Maharaj A, Burnell M. et al. Ovarian cancer population screening and mortality after long-term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet 2021; 397 (10290): 2182-2193 DOI: 10.1016/S0140-6736(21)00731-5.
- Daly MB, Pal T, Berry MP. et al; Genetic/familial high-risk assessment: breast, ovarian, and pancreatic, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2021; 19 (01) 77-102 DOI: 10.6004/jnccn.2021.0001.
- Atri M, Alabousi A, Reinhold C. et al; Expert Panel on Women's Imaging. ACR appropriateness criteria® clinically suspected adnexal mass, no acute symptoms. J Am Coll Radiol 2019; 16 (5S): S77-S93 DOI: 10.1016/j.jacr.2019.02.011.
- Schmidt S, Meuli RA, Achtari C, Prior JO. Peritoneal carcinomatosis in primary ovarian cancer staging: comparison between MDCT, MRI, and 18F-FDG PET/CT. Clin Nucl Med 2015; 40 (05) 371-377
- Liu J, Xu Y, Wang J. Ultrasonography, computed tomography and magnetic resonance imaging for diagnosis of ovarian carcinoma. Eur J Radiol 2007; 62 (03) 328-334
- Kang SK, Reinhold C, Atri M. et al; Expert Panel on Women's Imaging. ACR appropriateness criteria® staging and follow-up of ovarian cancer. J Am Coll Radiol 2018; 15 (5S): S198-S207 DOI: 10.1016/j.jacr.2018.03.015.
- Rockall A, Munari A, Avril N. New ways of assessing ovarian cancer response: metabolic imaging and beyond. Cancer Imaging 2012; 12 (02) 310-314 DOI: 10.1102/1470-7330.2012.9004.
- Vallius T, Hynninen J, Kemppainen J. et al. 18F-FDG-PET/CT based total metabolic tumor volume change during neoadjuvant chemotherapy predicts outcome in advanced epithelial ovarian cancer. Eur J Nucl Med Mol Imaging 2018; 45 (07) 1224-1232 DOI: 10.1007/s00259-018-3961-z.
- Winfield JM, Wakefield JC, Dolling D. et al. Diffusion-weighted MRI in advanced epithelial ovarian cancer: apparent diffusion coefficient as a response marker. Radiology 2019; 293 (02) 374-383 DOI: 10.1148/radiol.2019190545.
- Sebastian S, Lee SI, Horowitz NS. et al. PET-CT vs. CT alone in ovarian cancer recurrence. Abdom Imaging 2008; 33 (01) 112-118 DOI: 10.1007/s00261-007-9218-0.
- Bast Jr RC, Klug TL, St John E. et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med 1983; 309 (15) 883-887
- Dodge JE, Covens AL, Lacchetti C. et al; Gynecology Cancer Disease Site Group. Preoperative identification of a suspicious adnexal mass: a systematic review and meta-analysis. Gynecol Oncol 2012; 126 (01) 157-166
- Hewitt MJ, Anderson K, Hall GD. et al. Women with peritoneal carcinomatosis of unknown origin: Efficacy of image-guided biopsy to determine site-specific diagnosis. BJOG 2007; 114 (01) 46-50
- Fagotti A, Ferrandina G, Fanfani F. et al. A laparoscopy-based score to predict surgical outcome in patients with advanced ovarian carcinoma: a pilot study. Ann Surg Oncol 2006; 13 (08) 1156-1161
- Rutten MJ, van Meurs HS, van de Vrie R. et al. Laparoscopy to predict the result of primary cytoreductive surgery in patients with advanced ovarian cancer: a randomized controlled trial. J Clin Oncol 2017; 35 (06) 613-621
- Konstantinopoulos PA, Norquist B, Lacchetti C. et al. Germline and somatic tumor testing in epithelial ovarian cancer: ASCO guideline. J Clin Oncol 2020; 38 (11) 1222-1245
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin 2021; 71 (01) 7-33
- Buys SS, Partridge E, Black A. et al; PLCO Project Team. Effect of screening on ovarian cancer mortality: the prostate, lung, colorectal and ovarian (PLCO) cancer screening randomized controlled trial. JAMA 2011; 305 (22) 2295-2303 DOI: 10.1001/jama.2011.766.
- Grossman DC, Curry SJ, Owens DK. et al; US Preventive Services Task Force. Screening for ovarian cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018; 319 (06) 588-594 DOI: 10.1001/jama.2017.21926.
- Committee on Gynecologic Practice, Society of Gynecologic Oncology. Committee Opinion No. 716: the role of the obstetrician-gynaecologist in the early detection of epithelial ovarian cancer in women at average risk. Obstet Gynecol 2017; 130 (03) e146-e149
- Smith RA, Andrews KS, Brooks D. et al. Cancer screening in the United States, 2017: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin 2017; 67 (02) 100-121 DOI: 10.3322/caac.21392.
- Andreotti RF, Timmerman D, Strachowski LM. et al. O-RADS US risk stratification and management system: a consensus guideline from the ACR Ovarian-Adnexal Reporting and Data System Committee. Radiology 2020; 294 (01) 168-185 DOI: 10.1148/radiol.2019191150.
- Sadowski EA, Thomassin-Naggara I, Rockall A. et al. O-RADS MRI risk stratification system: guide for assessing adnexal lesions from the ACR O-RADS Committee. Radiology 2022; 303 (01) 35-47 DOI: 10.1148/radiol.204371.
- Hynninen J, Kemppainen J, Lavonius M. et al. A prospective comparison of integrated FDG-PET/contrast-enhanced CT and contrast-enhanced CT for pretreatment imaging of advanced epithelial ovarian cancer. Gynecol Oncol 2013; 131 (02) 389-394
- Koh JL, Yan TD, Glenn D, Morris DL. Evaluation of preoperative computed tomography in estimating peritoneal cancer index in colorectal peritoneal carcinomatosis. Ann Surg Oncol 2009; 16 (02) 327-333 DOI: 10.1245/s10434-008-0234-2.
- Low RN, Barone RM. Combined diffusion-weighted and gadolinium-enhanced MRI can accurately predict the peritoneal cancer index preoperatively in patients being considered for cytoreductive surgical procedures. Ann Surg Oncol 2012; 19 (05) 1394-1401
- Castellucci P, Perrone AM, Picchio M. et al. Diagnostic accuracy of 18F-FDG PET/CT in characterizing ovarian lesions and staging ovarian cancer: correlation with transvaginal ultrasonography, computed tomography, and histology. Nucl Med Commun 2007; 28 (08) 589-595 DOI: 10.1097/MNM.0b013e3281afa256.
- Nam EJ, Yun MJ, Oh YT. et al. Diagnosis and staging of primary ovarian cancer: correlation between PET/CT, Doppler US, and CT or MRI. Gynecol Oncol 2010; 116 (03) 389-394
- Mutch DG, Prat J. 2014 FIGO staging for ovarian, fallopian tube and peritoneal cancer. Gynecol Oncol 2014; 133 (03) 401-404
- Berek JS, Renz M, Kehoe S, Kumar L, Friedlander M. Cancer of the ovary, fallopian tube, and peritoneum: 2021 update. Int J Gynaecol Obstet 2021; 155 (Suppl 1): 61-85 DOI: 10.1002/ijgo.13878.
- Tawakol A, Abdelhafez YG, Osama A, Hamada E, El Refaei S. Diagnostic performance of 18F-FDG PET/contrast-enhanced CT versus contrast-enhanced CT alone for post-treatment detection of ovarian malignancy. Nucl Med Commun 2016; 37 (05) 453-460
- Gu P, Pan LL, Wu SQ, Sun L, Huang G. CA 125, PET alone, PET-CT, CT and MRI in diagnosing recurrent ovarian carcinoma: a systematic review and meta-analysis. Eur J Radiol 2009; 71 (01) 164-174
- Nakamoto Y, Saga T, Ishimori T. et al. Clinical value of positron emission tomography with FDG for recurrent ovarian cancer. Am J Roentgenol 2001; 176 (06) 1449-1454
- Salani R, Backes FJ, Fung MFK. et al. Posttreatment surveillance and diagnosis of recurrence in women with gynecologic malignancies: Society of Gynecologic Oncologists recommendations. Am J Obstet Gynecol 2011; 204 (06) 466-478
- Chi DS, Eisenhauer EL, Zivanovic O. et al. Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Gynecol Oncol 2009; 114 (01) 26-31 DOI: 10.1016/j.ygyno.2009.03.018.
- Elattar A, Bryant A, Winter-Roach BA, Hatem M, Naik R. Optimal primary surgical treatment for advanced epithelial ovarian cancer. Cochrane Database Syst Rev 2011; (08) CD007565 DOI: 10.1002/14651858.CD007565.pub2.
- Kehoe S, Hook J, Nankivell M. et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet 2015; 386 (9990): 249-257 DOI: 10.1016/S0140-6736(14)62223-6.
- Makar AP, Tropé CG, Tummers P, Denys H, Vandecasteele K. Advanced ovarian cancer: primary or interval debulking? Five categories of patients in view of the results of randomized trials and tumor biology: primary debulking surgery and interval debulking surgery for advanced ovarian cancer. Oncologist 2016; 21 (06) 745-754 DOI: 10.1634/theoncologist.2015-0239.
- Tewari KS, Burger RA, Enserro D. et al. Final overall survival of a randomized trial of bevacizumab for primary treatment of ovarian cancer. J Clin Oncol 2019; 37 (26) 2317-2328 DOI: 10.1200/JCO.19.01009.
- Moore K, Colombo N, Scambia G. et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 2018; 379 (26) 2495-2505 DOI: 10.1056/NEJMoa1810858.
- Rustin GJS. Follow-up with CA125 after primary therapy of advanced ovarian cancer has major implications for treatment outcome and trial performances and should not be routinely performed. Ann Oncol 2011; 22 (Suppl 8) viii45-viii48 DOI: 10.1093/annonc/mdr471.
- Esselen KM, Cronin AM, Bixel K. et al. Use of CA-125 tests and computed tomographic scans for surveillance in ovarian cancer. JAMA Oncol 2016; 2 (11) 1427-1433 DOI: 10.1001/jamaoncol.2016.1842.


 PDF
PDF  Views
Views  Share
Share

