Imaging spectrum of gastrointestinal stromal tumor
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2014; 35(02): 143-148
DOI: DOI: 10.4103/0971-5851.138964
Abstract
Gastrointestinal stromal tumors (GISTs) were first described by Clark and Mazur in 1983 for smooth muscle neoplasms of the gastrointestinal tract differentiating them from leiomyoma, leiomyosarcomas and neurogenic tumors. GISTs can arise from the bowel, peritoneum, omentum or retroperitoneum. This article reviews the computed tomography imaging features of primary GISTs, response to treatment and highlights data on predicting the outcome to chemotherapeutic drugs on imaging.
Publication History
Article published online:
19 July 2021
© 2014. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Gastrointestinal stromal tumors (GISTs) were first described by Clark and Mazur in 1983 for smooth muscle neoplasms of the gastrointestinal tract differentiating them from leiomyoma, leiomyosarcomas and neurogenic tumors. GISTs can arise from the bowel, peritoneum, omentum or retroperitoneum. This article reviews the computed tomography imaging features of primary GISTs, response to treatment and highlights data on predicting the outcome to chemotherapeutic drugs on imaging.
INTRODUCTION
Gastrointestinal stromal tumors (GISTs) are special group of tumors arising from interstitial cells of Cajal, that express a tyrosine kinase growth factor receptor, also called KIT protein-CD117 and CD34 found in chromosome 4. The expression of this unique protein allows unchecked growth of tumor, which is also resistance to apoptosis. These tumors are different from leiomyoma, leiomyoblastoma and leiomyosarcomas. Before 1983, GISTs were misdiagnosed as smooth muscle tumors because on light microscopy these tumors share many features.
DISCUSSION
Gastrointestinal stromal tumors most affects people of age group 40-70 years old. Male to female incidence is now considered to be equal. Statistically, between 5000 and 10,000 people develop this tumor worldwide with nearly 1000-2000 new cases/year in the United States. GISTs accounts for 1-3% of all gastrointestinal tumors. GISTs can involve the gastrointestinal tract anywhere from esophagus to anus — stomach (37-70%), small bowel (20-33%), duodenum (9%), anorectum (5-7%), colon (4%) and esophagus (<2 href="https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4152631/figure/F1/" target="figure" class="fig-table-link figpopup" rid-figpopup="F1" rid-ob="ob-F1" co-legend-rid="lgnd_F1" xss=removed>[Figures11--55].[1] Other atypical locations are omentum, mesentery and retroperitoneum [Figures [Figures66--88].[2] The histologic classification is based on the predominant cell type — spindle cell, epithelioid cell or mixed cell type. The spindle cell type accounts for about 75% of GISTs and is also the most common type of GIST at other sites.
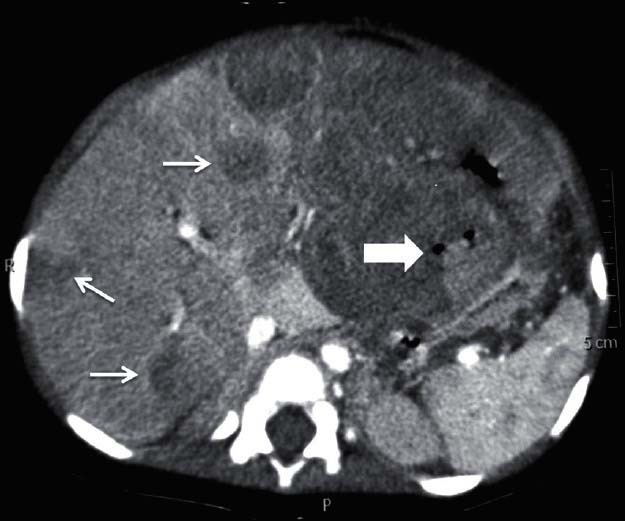
| Figure 1:Malignant stomach gastrointestinal stromal tumors in a 27-year-old female — axial contrast-enhanced computed tomography image showing large predominantly exophytic mass lesion arising from stomach (arrowhead) with multiple hypodense liver metastasis (arrows)
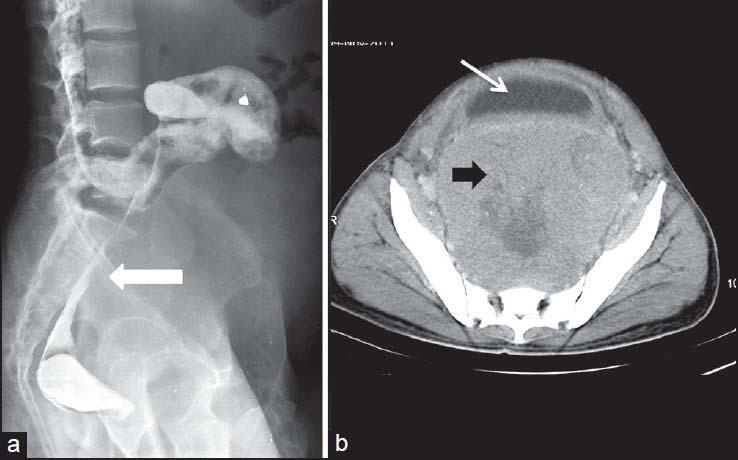
| Figure 5:Rectal gastrointestinal stromal tumors in a 49-year-old male — (a) barium enema spot image lateral view showing extrinsic impression on rectal wall (arrowhead) (b) axial contrast-enhanced computed tomography image showing heterogenously enhancing necrotic mass in pelvis (black arrowhead) displacing the urinary bladder (arrow) anteriorly
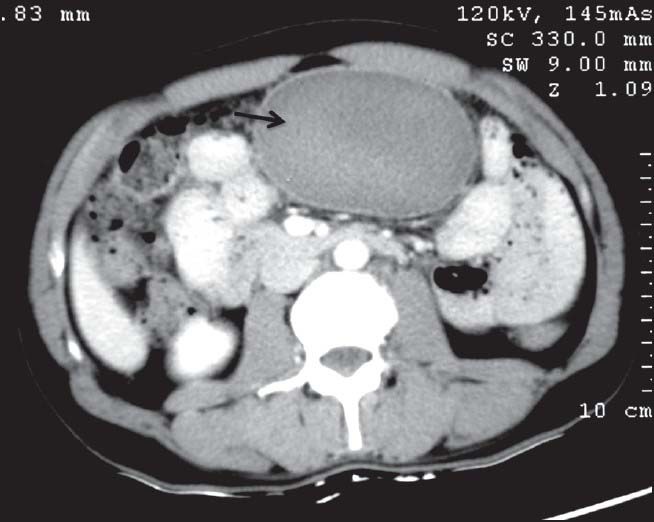
| Figure 6:Omental gastrointestinal stromal tumors in a 44-year-old male — axial contrast-enhanced computed tomography image showing well-defined homogenously enhancing mass (arrow) in the omentum displacing the bowel loops
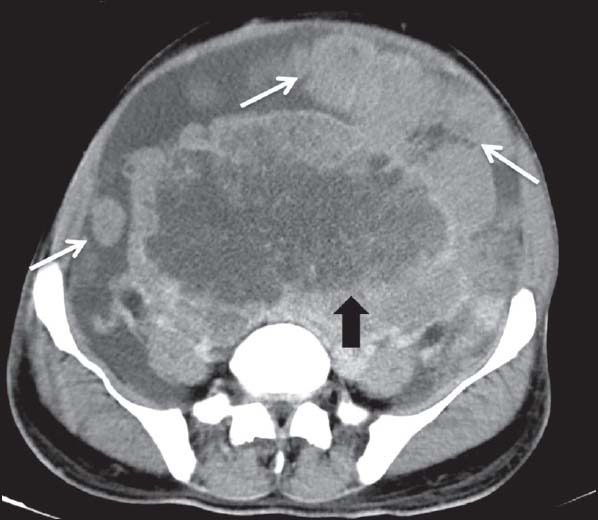
| Figure 8:Retroperitoneal gastrointestinal stromal tumors in 40-year-old male — axial contrast-enhanced computed tomography image showing large heterogeneously enhancing necrotic mass lesion (arrowhead) in the retroperitoneum with peritoneal deposits (arrow)
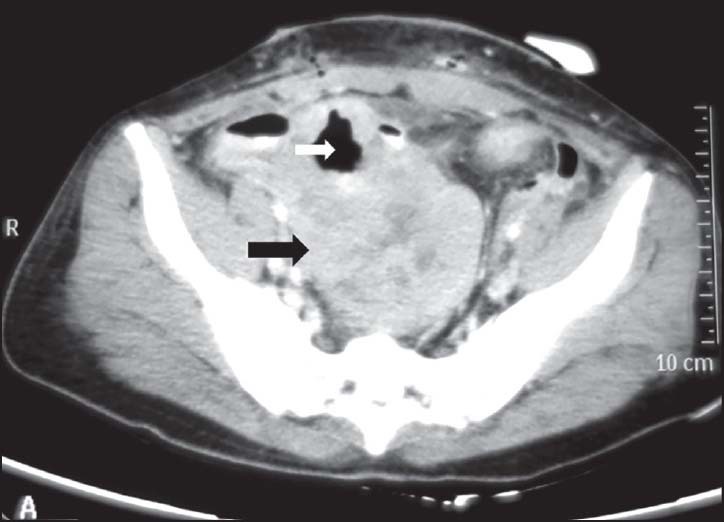
| Figure 2:Ileal gastrointestinal stromal tumor in a 45-year-old male — axial contrast-enhanced computed tomography image showing heterogeneously enhancing exophytic mass lesion (black arrowhead) arising from ileal loop causing mild aneurysmal dilatation (white arrowhead)
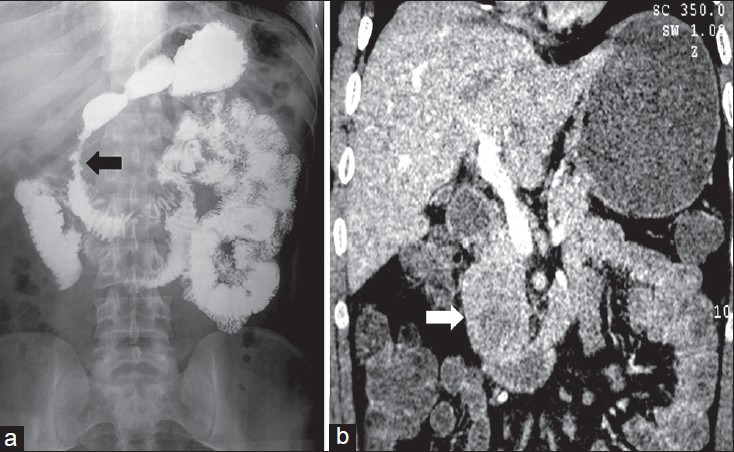
| Figure 3:Duodenal gastrointestinal stromal tumors in a 45-year-old male — (a) barium meal follow through spot image showing widening of C-loop of duodenum with mucosal irregularity (arrowhead) and ulcerations (b) coronal contrast-enhanced computed tomography image showing heterogeneously enhancing mass lesion in second part of duodenum (white arrowhead) having intraluminal as well as subserosal component without proximal obstruction
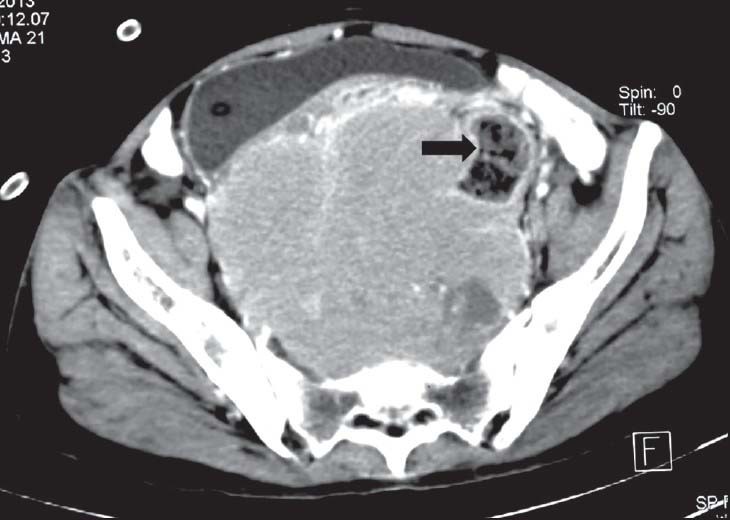
| Figure 4:Rectal gastrointestinal stromal tumors in a 50-year-old female contrast-enhanced computed tomography showing heterogeneously enhancing exophytic mass lesion arising from the rectal wall (arrowhead)
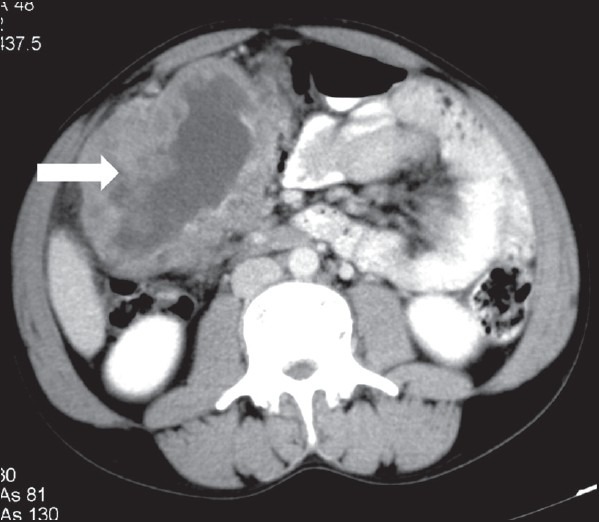
| Figure 7:Peritoneal gastrointestinal stromal tumors in a 40-yearold male — axial contrast-enhanced computed tomography image showing large heterogeneously enhancing mass lesion (arrowhead) with necrosis in the peritoneal cavity
On imaging, GISTs may be submucosal, intramural or subserosal. Findings on barium studies are often subtle than seen on computed tomography (CT) as majority of them are exophytic in nature. On CT, small GISTs are usually well-defined, solid mass showing homogenous enhancement [Figure 9]. Calcifications may occasionally be present. Large tumors show areas of hemorrhage, cystic/necrotic areas and heterogeneous enhancement. Neovascularity may be seen within the tumor after contrast administration [Figure 10]. Thrombosis of mesenteric vessels is uncommon. Rarely, these tumors may be entirely cystic [Figure 11]. Cavitory nature with air and contrast within the mass is suggestive of mucosal ulceration with fistulous communication of necrotic cavity with the bowel lumen [Figure 12].[3,4] Collection of air in the nondependent aspect of larger cavitating tumors with necrosis is known as the “Toricelli-Bernouilli” crescentic necrosis sign [Figure 13].[5] Uncommon features with GIST are presence of ascites, lymphadenopathy, intestinal obstruction and metastases in lungs [Figure 14]. Crucial role lies in differentiation of benign versus malignant GISTs. Features associated with poor prognosis include distal location, size and high mitotic activity with the exception of esophageal GISTs.[6] Benign lesions are usually <2>5 cm.[7] Smaller tumors should be classified as at lower risk for malignancy rather than as benign. Tumor necrosis, cystic change, nuclear atypia, tumor vascularity, and degree of staining for CD117 are unreliable predictors of malignancy.[8] Metastases are most common in the liver, mesentery, and peritoneum [Figure 15].[9,10] Calcification is usually seen in metastases after specific chemotherapy.
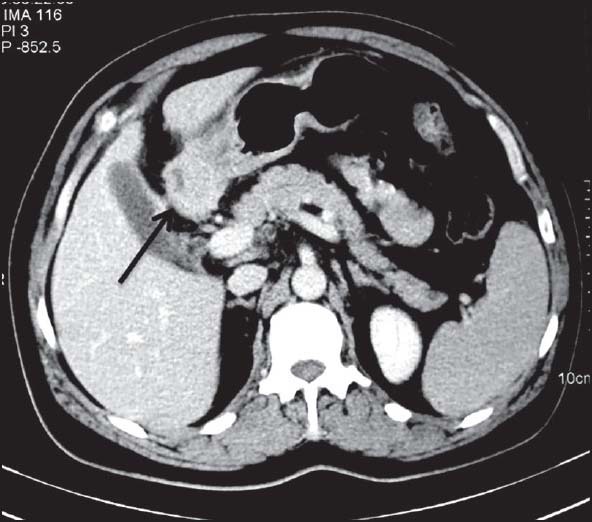
| Figure 9:Pyloric gastrointestinal stromal tumors in a 47-year-old male — axial contrast-enhanced computed tomography image showing relatively homogenously enhancing mass (arrow) in pylorus of stomach with necrotic center having submucosal as well as intramural component
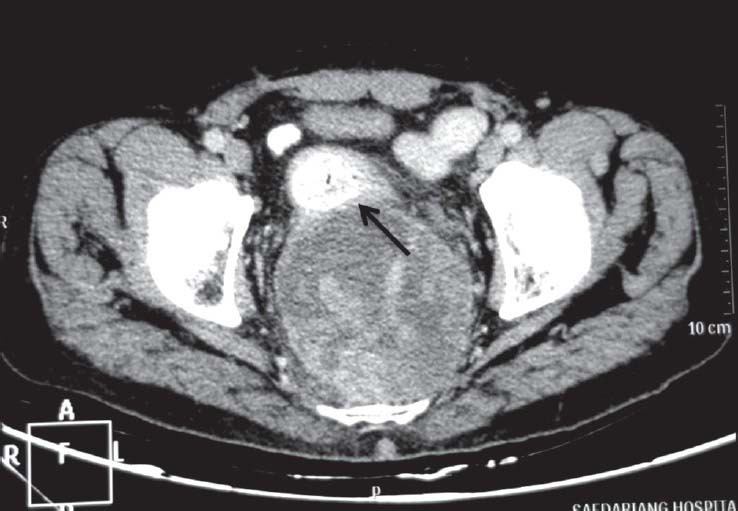
| Figure 10:Rectal gastrointestinal stromal tumors in 68-year-old male — axial contrast-enhanced computed tomography image showing exophytic heterogeneously enhancing mass lesion arising from rectal wall (arrow) with areas of necrosis
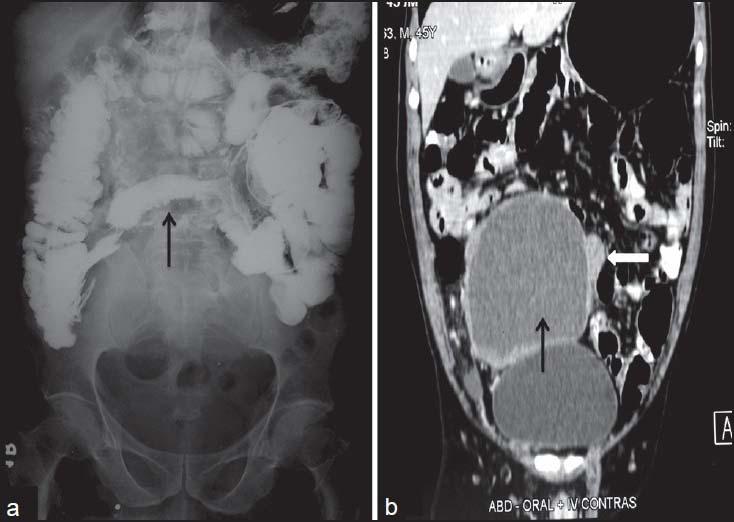
| Figure 11:Cystic gastrointestinal stromal tumors in a 51-yearold male — (a) barium meal follow through spot image showing displacement of terminal ileal loops (arrow) with extrinsic impression and obtuse angles (b) coronal contrast-enhanced computed tomography image showing cystic mass (arrow) arising from the ileal loop (arrowhead)
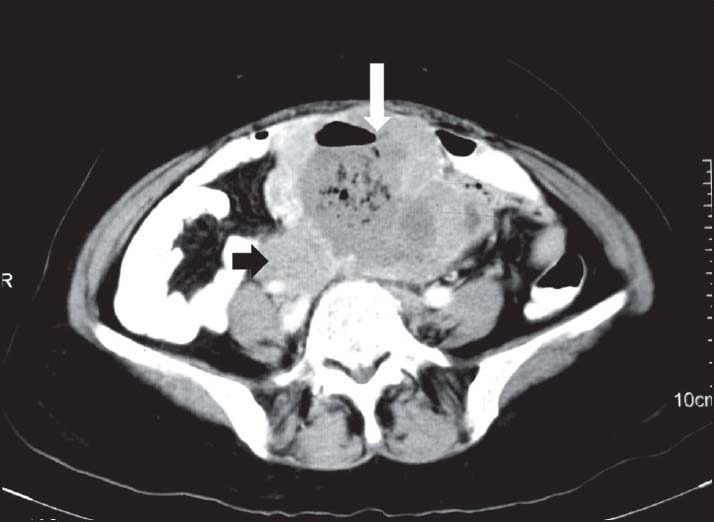
| Figure 12:Malignant small bowel gastrointestinal stromal tumors with lymphadenopathy — axial contrast-enhanced computed tomography image showing large cavitating exophytic mass lesion arising from small bowel (white arrowhead) with right iliac lymph node (black arrowhead)
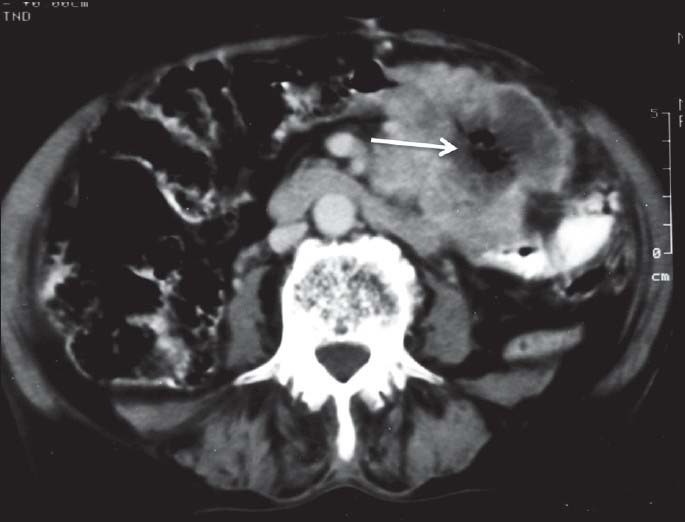
| Figure 13:Small bowel gastrointestinal stromal tumors in a 70-yearold female — axial contrast-enhanced computed tomography image showing exophytic mass arising from jejunal loop with necrosis and air within the tumor (arrow) — “Toricelli-Bernouilli” sign
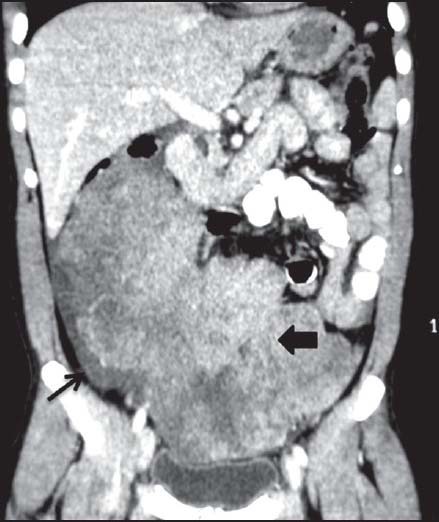
| Figure 14:Peritoneal gastrointestinal stromal tumors with mild ascites in 52-year-old male — coronal contrast-enhanced computed tomography image showing heterogenously enhancing peritoneal mass (arrowhead) with minimal ascites (arrow)
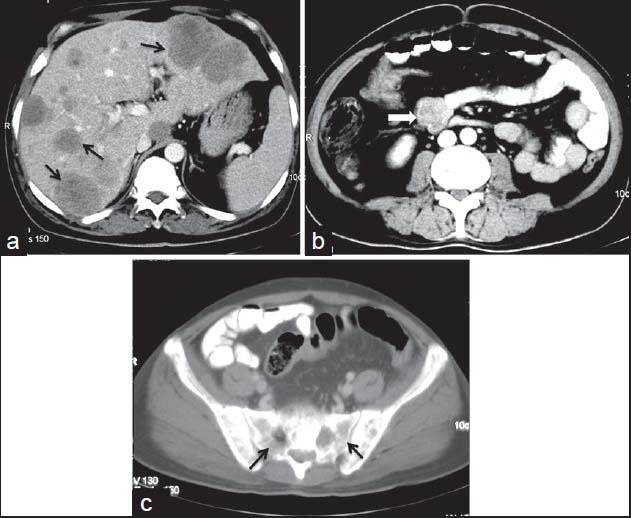
| Figure 15:Malignant small bowel gastrointestinal stromal tumors in 45-year-old male — (a) contrast-enhanced computed tomography (CECT) showing multiple liver metastases (arrows) (b) axial CECT image showing heterogenous mass lesion with exophytic component (arrowhead) arising from jejunal loop (c) CECT bone window showing bony metastases (arrows)
Complete surgical excision is the treatment of choice. Unlike carcinomas, resection of GISTs does not require wide bowel excision or lymphadenectomy as these tumors usually do not show lymph node metastases.[8] However, despite apparently complete resection with clear margins, the recurrence rate is high. Chemotherapy with imatinib mesylate, a tyrosine kinase inhibitor has been found to be highly effective in treatment of metastatic GIST. Imatinib mesylate has revolutionized the management of GIST. Now it is often used as the first-line treatment for unresectable, metastatic, or recurrent GIST. Although complete responses are rare, a large majority of patients with metastatic or inoperable GIST have either a partial response or disease stabilization after starting imatinib. Median survival rates have gone from <2>5 years since the advent of imatinib therapy.[11] Role of imaging also lies in response evaluation of patients treated with imatinib. Response to imatinib is characterized by decreased enhancement, resolution of the enhancing tumor nodules and a decrease in tumor neovascularity [Figures [Figures1616--20].20]. These changes are usually seen within 1-month of initiation of chemotherapy. Initially, tumors may enlarge during treatment due to intratumoral hemorrhage and myxoid degeneration. Enlargement with an overall decrease in tumor enhancement suggests a favorable response. Presence of new enhancing nodules within the tumor is consistent with recurrence.[12]
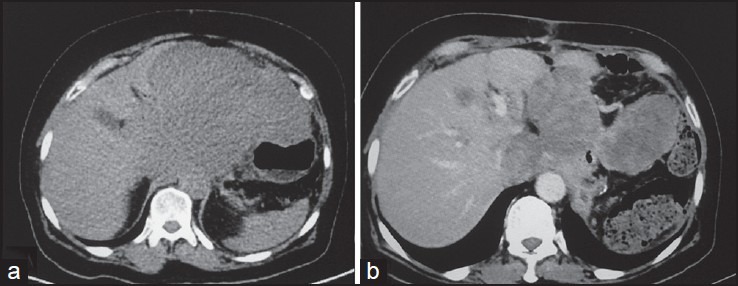
| Figure 16:Malignant metastatic stomach gastrointestinal stromal tumors posttreatment — (a) Axial noncontrast computed tomography scan showing heterogeneous mass arising from stomach and abutting the liver (b) posttreatment the mass shows heterogeneous enhancement with significant decrease in size
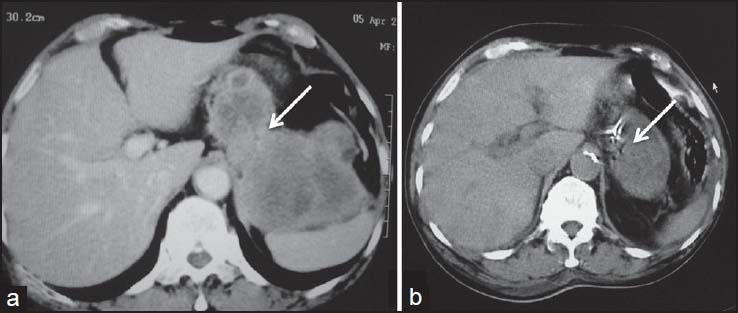
| Figure 20:Stomach gastrointestinal stromal tumors (GIST) postsurgery — (a) axial contrast-enhanced computed tomography showing heterogenously enhancing (arrow) stomach GIST (b) postsurgery, there is significant decrease in size with residual tumor showing minimal enhancement (arrow)
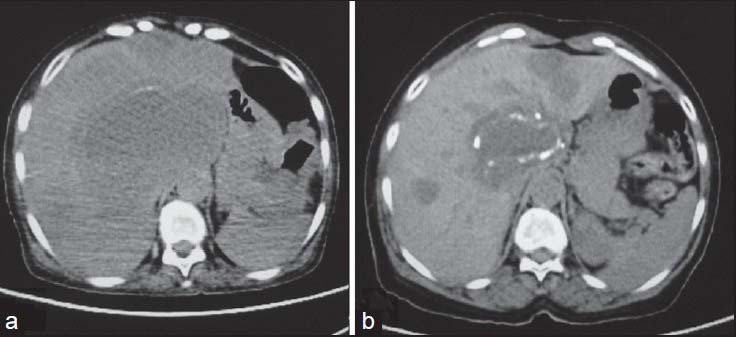
| Figure 17:Metastatic gastrointestinal stromal tumors posttreatment — (a) axial noncontrast computed tomography scan showing multiple metastasis in liver (b) Posttreatment calcification is seen within the metastatic lesions with reduction in size
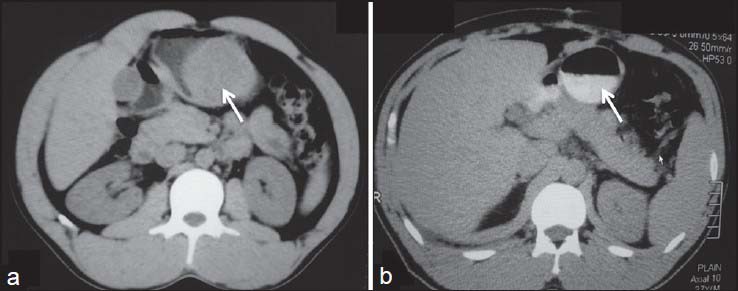
| Figure 18:Submucosal stomach gastrointestinal stromal tumors (GISTs) postsurgery — (a) axial contrast-enhanced computed tomography showing homogenously enhancing (arrow) stomach GIST (b) postsurgery, there is complete resection of the tumor with no evidence of recurrence (arrow)
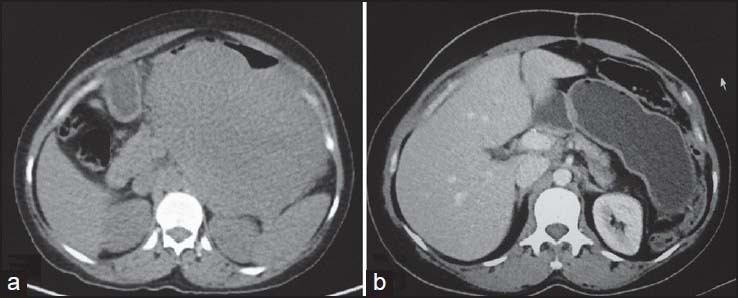
| Figure 19:Submucosal stomach gastrointestinal stromal tumors (GISTs) postsurgery — (a) axial contrast-enhanced computed tomography showing homogenously enhancing stomach GIST (b) Postsurgery, there is complete resection of the tumor with no evidence of recurrence
Choi et al. have demonstrated that a decrease in tumor size of >10% or a decrease in tumor density of >15% had a sensitivity of 97% and a specificity of 100% in detecting patients with good response to treatment with imatinib evaluated by positron emission tomography (PET)-CT in metastatic GIST.[13] A short-term follow-up by CT scan can be done at 1-month if PET is not available. GISTs are associated with Carney's triad, adrenocortical adenoma, ulcerative colitis, esophageal leiomyoma, and neurofibromatosis.[10]
CONCLUSION
Gastrointestinal stromal tumors are a unique and special group of mesenchymal tumors that predominantly exhibit an altered oncogene — kit (CD117). With the availability of specific molecular therapy, radiologists can often timely predict the correct diagnosis of a large exophytic bowel mass, which may show necrosis or hemorrhage. Radiologic appearances can change drastically after therapy and knowledge of such imaging features is beneficial to oncologists in managing these patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- Miettinen M, Sarlomo-Rikala M, Lasota J. Gastrointestinal stromal tumours. Ann Chir Gynaecol 1998;87:278-81.
- Sripathi S, Rajagopal K, Srivastava RK, Ayachit A. CT features, mimics and atypical presentations of gastrointestinal stromal tumor (GIST). Indian J Radiol Imaging 2011;21:176-81.
- King DM. The radiology of gastrointestinal stromal tumours (GIST). Cancer Imaging 2005;5:150-6.
- Patnaik S, Jyotsnarani Y, Rammurti S. Radiological features of metastatic gastrointestinal stromal tumors. J Clin Imaging Sci 2012;2:43.
- Sandrasegaran K, Rajesh A, Rydberg J, Rushing DA, Akisik FM, Henley JD. Gastrointestinal stromal tumors: Clinical, radiologic, and pathologic features. AJR Am J Roentgenol 2005;184:803-11.
- Rudolph P, Chiaravalli AM, Pauser U, Oschlies I, Hillemanns M, Gobbo M, et al. Gastrointestinal mesenchymal tumors - immunophenotypic classification and survival analysis. Virchows Arch 2002;441:238-48.
- DeMatteo RP. The GIST of targeted cancer therapy: A tumor (gastrointestinal stromal tumor), a mutated gene (c-kit), and a molecular inhibitor (STI571). Ann Surg Oncol 2002;9:831-9.
- Berman J, O′Leary TJ. Gastrointestinal stromal tumor workshop. Hum Pathol 2001;32:578-82.
- Levy AD, Remotti HE, Thompson WM, Sobin LH, Miettinen M. Gastrointestinal stromal tumors: Radiologic features with pathologic correlation. Radiographics 2003;23:283-304, 456.
- Ludwig DJ, Traverso LW. Gut stromal tumors and their clinical behavior. Am J Surg 1997;173:390-4.
- Blanke CD, Demetri GD, von Mehren M, Heinrich MC, Eisenberg B, Fletcher JA, et al. Long-term results from a randomized phase II trial of standard-versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol 2008;26:620-5.
- Hong X, Choi H, Loyer EM, Benjamin RS, Trent JC, Charnsangavej C. Gastrointestinal stromal tumor: Role of CT in diagnosis and in response evaluation and surveillance after treatment with imatinib. Radiographics 2006;26:481-95.
- Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: Proposal of new computed tomography response criteria. J Clin Oncol 2007;25:1753-9.

| Figure 1:Malignant stomach gastrointestinal stromal tumors in a 27-year-old female — axial contrast-enhanced computed tomography image showing large predominantly exophytic mass lesion arising from stomach (arrowhead) with multiple hypodense liver metastasis (arrows)

| Figure 5:Rectal gastrointestinal stromal tumors in a 49-year-old male — (a) barium enema spot image lateral view showing extrinsic impression on rectal wall (arrowhead) (b) axial contrast-enhanced computed tomography image showing heterogenously enhancing necrotic mass in pelvis (black arrowhead) displacing the urinary bladder (arrow) anteriorly

| Figure 6:Omental gastrointestinal stromal tumors in a 44-year-old male — axial contrast-enhanced computed tomography image showing well-defined homogenously enhancing mass (arrow) in the omentum displacing the bowel loops

| Figure 8:Retroperitoneal gastrointestinal stromal tumors in 40-year-old male — axial contrast-enhanced computed tomography image showing large heterogeneously enhancing necrotic mass lesion (arrowhead) in the retroperitoneum with peritoneal deposits (arrow)

| Figure 2:Ileal gastrointestinal stromal tumor in a 45-year-old male — axial contrast-enhanced computed tomography image showing heterogeneously enhancing exophytic mass lesion (black arrowhead) arising from ileal loop causing mild aneurysmal dilatation (white arrowhead)

| Figure 3:Duodenal gastrointestinal stromal tumors in a 45-year-old male — (a) barium meal follow through spot image showing widening of C-loop of duodenum with mucosal irregularity (arrowhead) and ulcerations (b) coronal contrast-enhanced computed tomography image showing heterogeneously enhancing mass lesion in second part of duodenum (white arrowhead) having intraluminal as well as subserosal component without proximal obstruction

| Figure 4:Rectal gastrointestinal stromal tumors in a 50-year-old female contrast-enhanced computed tomography showing heterogeneously enhancing exophytic mass lesion arising from the rectal wall (arrowhead)

| Figure 7:Peritoneal gastrointestinal stromal tumors in a 40-yearold male — axial contrast-enhanced computed tomography image showing large heterogeneously enhancing mass lesion (arrowhead) with necrosis in the peritoneal cavity

| Figure 9:Pyloric gastrointestinal stromal tumors in a 47-year-old male — axial contrast-enhanced computed tomography image showing relatively homogenously enhancing mass (arrow) in pylorus of stomach with necrotic center having submucosal as well as intramural component

| Figure 10:Rectal gastrointestinal stromal tumors in 68-year-old male — axial contrast-enhanced computed tomography image showing exophytic heterogeneously enhancing mass lesion arising from rectal wall (arrow) with areas of necrosis

| Figure 11:Cystic gastrointestinal stromal tumors in a 51-yearold male — (a) barium meal follow through spot image showing displacement of terminal ileal loops (arrow) with extrinsic impression and obtuse angles (b) coronal contrast-enhanced computed tomography image showing cystic mass (arrow) arising from the ileal loop (arrowhead)

| Figure 12:Malignant small bowel gastrointestinal stromal tumors with lymphadenopathy — axial contrast-enhanced computed tomography image showing large cavitating exophytic mass lesion arising from small bowel (white arrowhead) with right iliac lymph node (black arrowhead)

| Figure 13:Small bowel gastrointestinal stromal tumors in a 70-yearold female — axial contrast-enhanced computed tomography image showing exophytic mass arising from jejunal loop with necrosis and air within the tumor (arrow) — “Toricelli-Bernouilli” sign

| Figure 14:Peritoneal gastrointestinal stromal tumors with mild ascites in 52-year-old male — coronal contrast-enhanced computed tomography image showing heterogenously enhancing peritoneal mass (arrowhead) with minimal ascites (arrow)

| Figure 15:Malignant small bowel gastrointestinal stromal tumors in 45-year-old male — (a) contrast-enhanced computed tomography (CECT) showing multiple liver metastases (arrows) (b) axial CECT image showing heterogenous mass lesion with exophytic component (arrowhead) arising from jejunal loop (c) CECT bone window showing bony metastases (arrows)

| Figure 16:Malignant metastatic stomach gastrointestinal stromal tumors posttreatment — (a) Axial noncontrast computed tomography scan showing heterogeneous mass arising from stomach and abutting the liver (b) posttreatment the mass shows heterogeneous enhancement with significant decrease in size

| Figure 20:Stomach gastrointestinal stromal tumors (GIST) postsurgery — (a) axial contrast-enhanced computed tomography showing heterogenously enhancing (arrow) stomach GIST (b) postsurgery, there is significant decrease in size with residual tumor showing minimal enhancement (arrow)

| Figure 17:Metastatic gastrointestinal stromal tumors posttreatment — (a) axial noncontrast computed tomography scan showing multiple metastasis in liver (b) Posttreatment calcification is seen within the metastatic lesions with reduction in size

| Figure 18:Submucosal stomach gastrointestinal stromal tumors (GISTs) postsurgery — (a) axial contrast-enhanced computed tomography showing homogenously enhancing (arrow) stomach GIST (b) postsurgery, there is complete resection of the tumor with no evidence of recurrence (arrow)

| Figure 19:Submucosal stomach gastrointestinal stromal tumors (GISTs) postsurgery — (a) axial contrast-enhanced computed tomography showing homogenously enhancing stomach GIST (b) Postsurgery, there is complete resection of the tumor with no evidence of recurrence
References
- Miettinen M, Sarlomo-Rikala M, Lasota J. Gastrointestinal stromal tumours. Ann Chir Gynaecol 1998;87:278-81.
- Sripathi S, Rajagopal K, Srivastava RK, Ayachit A. CT features, mimics and atypical presentations of gastrointestinal stromal tumor (GIST). Indian J Radiol Imaging 2011;21:176-81.
- King DM. The radiology of gastrointestinal stromal tumours (GIST). Cancer Imaging 2005;5:150-6.
- Patnaik S, Jyotsnarani Y, Rammurti S. Radiological features of metastatic gastrointestinal stromal tumors. J Clin Imaging Sci 2012;2:43.
- Sandrasegaran K, Rajesh A, Rydberg J, Rushing DA, Akisik FM, Henley JD. Gastrointestinal stromal tumors: Clinical, radiologic, and pathologic features. AJR Am J Roentgenol 2005;184:803-11.
- Rudolph P, Chiaravalli AM, Pauser U, Oschlies I, Hillemanns M, Gobbo M, et al. Gastrointestinal mesenchymal tumors - immunophenotypic classification and survival analysis. Virchows Arch 2002;441:238-48.
- DeMatteo RP. The GIST of targeted cancer therapy: A tumor (gastrointestinal stromal tumor), a mutated gene (c-kit), and a molecular inhibitor (STI571). Ann Surg Oncol 2002;9:831-9.
- Berman J, O′Leary TJ. Gastrointestinal stromal tumor workshop. Hum Pathol 2001;32:578-82.
- Levy AD, Remotti HE, Thompson WM, Sobin LH, Miettinen M. Gastrointestinal stromal tumors: Radiologic features with pathologic correlation. Radiographics 2003;23:283-304, 456.
- Ludwig DJ, Traverso LW. Gut stromal tumors and their clinical behavior. Am J Surg 1997;173:390-4.
- Blanke CD, Demetri GD, von Mehren M, Heinrich MC, Eisenberg B, Fletcher JA, et al. Long-term results from a randomized phase II trial of standard-versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol 2008;26:620-5.
- Hong X, Choi H, Loyer EM, Benjamin RS, Trent JC, Charnsangavej C. Gastrointestinal stromal tumor: Role of CT in diagnosis and in response evaluation and surveillance after treatment with imatinib. Radiographics 2006;26:481-95.
- Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: Proposal of new computed tomography response criteria. J Clin Oncol 2007;25:1753-9.


 PDF
PDF  Views
Views  Share
Share

