Impact of Sarcopenia on Head and Neck Cancer Treatment: A Review of Literature
CC BY 4.0 · Indian J Med Paediatr Oncol 2023; 44(04): 391-397
DOI: OI: 10.1055/s-0043-1768690
Abstract
The overall outcome of head and neck cancer (HNC) patients undergoing any treatment modality may significantly depend upon their general nutritional condition. Poor nutritional status leading to sarcopenia may be a negative prognostic factor in determining the outcome of HNC patients. PubMed database was searched to identify studies published between 2015 and 2022. All studies reporting the index for sarcopenia as well as its effect on HNC were included. This narrative review was conducted to specifically evaluate the impact of sarcopenia on HNC patients undergoing surgery/ free flap reconstruction/ adjuvant treatment. In oncology, computed tomography assessment of skeletal mass at C3 and L3 is the most suitable index to detect sarcopenia. From the articles yielded, the prevalence rate of sarcopenia ranges from 6 to 70%-worldwide. Indian population presents with a significantly higher rate of 31.5%-sarcopenia HNC patients. Sarcopenic patients have an increased propensity for surgical site infections, as high as 24.6%-owing to the reduced skeletal muscle mass. These patients are also prone to have frequent breaks during radiation treatment of more than 1 week and increased chemotherapy-related toxicities. Further, sarcopenic individuals tend to have higher Ryle's tube dependency of more than 90 days. Sarcopenic patients undergoing surgery have a poor overall survival (OS) and disease-free survival (DFS). In terms of hazards ratio, sarcopenic patients have 1.96 times poor OS and 2.00 times poor DFS when compared to normal individuals who undergo HNC surgery. Sarcopenia is an indispensable part of cancer ailment and it is an independent factor negatively influencing DFS and OS. Thus, nutritional strategy needs to be developed to mitigate sarcopenic effects, especially in the Indian population in preoperative setting.
Keywords
sarcopenia - head and neck cancer - chemotherapy - radiation - overall survival - sarcopenic indexPublication History
Article published online:
08 May 2023
© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
The overall outcome of head and neck cancer (HNC) patients undergoing any treatment modality may significantly depend upon their general nutritional condition. Poor nutritional status leading to sarcopenia may be a negative prognostic factor in determining the outcome of HNC patients. PubMed database was searched to identify studies published between 2015 and 2022. All studies reporting the index for sarcopenia as well as its effect on HNC were included. This narrative review was conducted to specifically evaluate the impact of sarcopenia on HNC patients undergoing surgery/ free flap reconstruction/ adjuvant treatment. In oncology, computed tomography assessment of skeletal mass at C3 and L3 is the most suitable index to detect sarcopenia. From the articles yielded, the prevalence rate of sarcopenia ranges from 6 to 70%-worldwide. Indian population presents with a significantly higher rate of 31.5%-sarcopenia HNC patients. Sarcopenic patients have an increased propensity for surgical site infections, as high as 24.6%-owing to the reduced skeletal muscle mass. These patients are also prone to have frequent breaks during radiation treatment of more than 1 week and increased chemotherapy-related toxicities. Further, sarcopenic individuals tend to have higher Ryle's tube dependency of more than 90 days. Sarcopenic patients undergoing surgery have a poor overall survival (OS) and disease-free survival (DFS). In terms of hazards ratio, sarcopenic patients have 1.96 times poor OS and 2.00 times poor DFS when compared to normal individuals who undergo HNC surgery. Sarcopenia is an indispensable part of cancer ailment and it is an independent factor negatively influencing DFS and OS. Thus, nutritional strategy needs to be developed to mitigate sarcopenic effects, especially in the Indian population in preoperative setting.
Keywords
sarcopenia - head and neck cancer - chemotherapy - radiation - overall survival - sarcopenic index
Introduction
Head and neck cancer (HNC) is one of the most common malignancies in India, predominantly affecting the males.[1] [2] In 2020, India estimated nearly 1, 35,929 (10.3%) new oral cavity cancer cases as per the Globocan data.[3] Surgery has been the mainstay treatment modality and well-established standard of care for HNC.[4] [5] However, surgical procedures are lengthy and result in deformities, often followed by reduced food intake leading to nutritional deficiencies and weight loss. Additionally, a shift in paradigm has been observed for the treatment of locally advanced HNC cases utilizing radiotherapy (RT) and concurrent chemotherapy in adjuvant settting.[6] [7] Nonetheless, adjuvant treatment leads to remarkable toxicities such as nausea, vomiting, mucositis, dysphagia, and dermatitis, making maintenance of adequate nutrition a challenge.[8] Thus, knowing nutritional status is highly essential prior to such intense treatment regime during the management of HNC. Given the emerging impact of sarcopenia in the overall survival (OS) of HNC patients, this review was aimed to analyze the mechanism of action and assess the effect of low skeletal muscle mass (SMM) on surgical and postoperative complications in head and neck oncosurgery patients. In this study, we intended to review the literature for incidence of sarcopenia in HNC, mechanism of action, prognostic impact of sarcopenia on various treatment procedures including surgery, radiation, and chemotherapy.
Materials and Methods
PubMed database was searched to identify studies reporting the outcome of sarcopenia in HNC patients. All articles published from January 2015 to March 2022 were searched for this narrative review. The subsequent search terms were used: “Sarcopenia,” and “HNC” in conjunction with “surgery,” “free flap reconstruction,” “postoperative complications,” “overall survival,” “disease free survival,” or “adjuvant treatment,” “chemotherapy,” “sarcopenia index.” Boolean operators (NOT, AND, OR) were also used in succession to modify the search. Additionally, the references of all studies were also searched individually for any additional publications. Only studies in English language, full text publications, and those establishing the impact of sarcopenia in HNC in terms of surgery, OS, disease-free survival (DFS), adjuvant radiation, and chemotherapy were deemed eligible to be included in this review. Case reports, pediatric studies, and any cancer apart from HNC were excluded. The literature search was screened by two authors (HS and KBT) and any differences were sorted in consultation with third author (MB). Each study was assessed for afore mentioned inclusion criteria. The data was extracted by two different authors (HS and KBT) independently. The extracted data included first author, study designs, index to measure sarcopenia, and the criteria assessed.
What Is Sarcopenia?
Sarcopenia is defined as advanced and generalized loss of skeletal muscle with compromise in muscle strength as well as physical function.[9] [10] Nutritional status, including muscle mass, may play a crucial role in determining the overall response of the patient to the subjected treatment. Current literature in general has demonstrated sarcopenia to be a positive predictor of increased postsurgical complication.[9] Sarcopenia, also referred as loss of SMM, has been defined as an independent risk factor of both surgical and adjuvant treatment outcomes of cancer patients.[10] The definition proposed by European Working Group on Sarcopenia in Older People is most popular and stresses on physical strength, mass, and strength of the muscle.[11] The frequency of sarcopenia in patients with HNC reported in literature is as high as 71%, which may vary depending on geographic region and index used to calibrate sarcopenia.[12] Indian population itself presents with a sarcopenic prevalence of alarmingly high as 31.5%.[13] [14]
Reports of sarcopenia causing higher incidence of postoperative complications is well documented, and attributes significantly to chemotherapy related toxicity, longer hospital stays and lower survival outcomes.[10] However, data on sarcopenic patients undergoing HNC management is lacking.[9] [10] [15] In the few studies that highlighted the relationship of sarcopenia on survival of HNC patients was only guided radiologically assessed low SMM was used to define sarcopenia.[15]
Mechanism of Action
Tumor microenvironment, a recent concept consists of inflammatory markers involving inflammatory cells, cytokines and chemokines which induces carcinogenesis.[16] The exact pathogenesis of sarcopenia and its influence on the survival outcomes of HNC patients remains to be elucidated. Cancer progression is characterized by systemic inflammatory response (SIR), which tremendously exerts catabolic effects on the host metabolism to cause muscle breakdown leading to SIR cascade is characterized by cachexia and local inflammation.[17] This SIR in turn leads to further muscle breakdown and increased release of pro-inflammatory cytokines such as interleulin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and transforming growth factor beta receptor.[18] Hence, we focused to provide with a simple flowchart ([Fig. 1]) to understand the factors conducive to cancer progression as well as those associated with sarcopenia, thereby suggesting the interlinking negative synergetic prognosis factor in the survival outcomes of HNC patients.
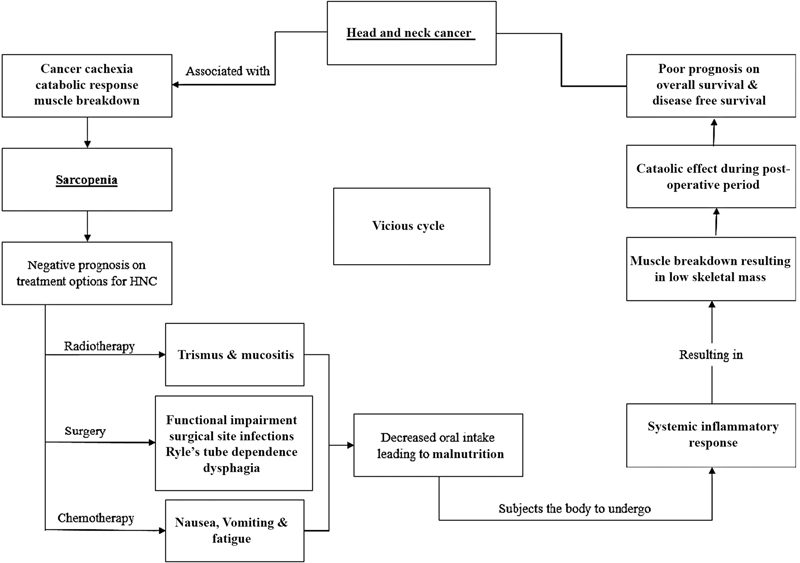
| Fig 1 : Inter-relation of sarcopenia on treatment of head and neck cancers (HNC).
How to Measure Sarcopenia
Till date, there is no consensus on a specific sarcopenic assessment method that can be incorporated in routine clinical practice. Therefore, we extrapolated the most common indices used from the literature for determining the SMM. Various tools for sarcopenia case finding and for measurement of muscle strength, muscle mass, and physical performance in clinical practice and in research are described in [Table 1], while various studies stating the cutoff values of the indices used in the literature are described in [Table 2].[19] [20] [21] [22] [23] [24] [25] [26] [27] [28]
|
Sr. no. |
Author |
Year |
Variable |
Index |
Cutoff Value |
|---|---|---|---|---|---|
|
1 |
Malmstrom et al[19] |
2016 |
Questionnaire |
SARC-F |
Score ≥ 4–better outcome |
|
2 |
Dodds et al[20] |
2014 |
Muscle strength |
Grip strength |
<27> <16> |
|
3 |
Studenski et al [21] |
2014 |
Muscle quantity |
ASMM by DXA |
<20> <15> |
|
4 |
Yamada et al[22] |
2017 |
Muscle quantity |
SMM with BIA |
6.8 kg/m2 for men 5.7 kg/m2 for women |
|
5 |
Cruz-Jentoft et al[11] |
2010 |
Physical performance |
Gait speed |
≤0.8 m/s |
|
6 |
Pavasini et al[23] |
2016 |
Physical performance |
SPPB |
≤8 point score |
|
7 |
Bischoff et al[24] |
2003 |
Physical performance |
TUG |
≥20 s |
|
8 |
Shanakaran et al[25] |
2018 |
Specific biomarkers |
Creatine dilution test[a] |
37 ± 10 kg for men 23 ± 4 kg for women |
|
9 |
Jung et al[26] |
2020 |
Radiographic measurement |
SMI at L3 |
52.4 for male 38.5 for female |
|
10 |
van Rijn-Dekker et al[27] |
2020 |
Radiographic measurement |
SMI at C3 |
42.4 for male 30.6 for female |
|
11 |
Yoshimura et al[28] |
2020 |
Radiographic measurement |
PMI |
6.05 for male 5.097 for female |
References
- Vigneswaran N, Williams MD. Epidemiologic trends in head and neck cancer and aids in diagnosis. Oral Maxillofac Surg Clin North Am 2014; 26 (02) 123-141
- Kulkarni MR. Head and neck cancer burden in India. Int J Head Neck Surg 2013; 4 (01) 29-35
- The Global Cancer Observatory - March, 2021. Accessed April 18, 2023 at: https://gco.iarc.fr
- Lo Nigro C, Denaro N, Merlotti A, Merlano M. Head and neck cancer: improving outcomes with a multidisciplinary approach. Cancer Manag Res 2017; 9: 363-371
- Anand AK, Agarwal JP, D'Cruz A. et al. Evolving multidisciplinary treatment of squamous cell carcinoma of the head and neck in India ✰ . Cancer Treat Res Commun 2021; 26: 100269
- Bonner JA, Harari PM, Giralt J. et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 2006; 354 (06) 567-578
- Pignon JP, le Maître A, Maillard E, Bourhis J. MACH-NC Collaborative Group. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol 2009; 92 (01) 4-14
- Ganju RG, Morse R, Hoover A, TenNapel M, Lominska CE. The impact of sarcopenia on tolerance of radiation and outcome in patients with head and neck cancer receiving chemoradiation. Radiother Oncol 2019; 137: 117-124
- Alwani MM, Jones AJ, Novinger LJ. et al. Impact of sarcopenia on outcomes of autologous head and neck free tissue reconstruction. J Reconstr Microsurg 2020; 36 (05) 369-378
- Ansari E, Chargi N, van Gemert JTM. et al. Low skeletal muscle mass is a strong predictive factor for surgical complications and a prognostic factor in oral cancer patients undergoing mandibular reconstruction with a free fibula flap. Oral Oncol 2020; 101: 104530
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM. et al; European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on Sarcopenia in older people. Age Ageing 2010; 39 (04) 412-423
- Takenaka Y, Takemoto N, Oya R, Inohara H. Prognostic impact of sarcopenia in patients with head and neck cancer treated with surgery or radiation: a meta-analysis. PLoS One 2021; 16 (10) e0259288
- Chauhan NS, Samuel SR, Meenar N, Saxena PP, Keogh JWL. Sarcopenia in male patients with head and neck cancer receiving chemoradiotherapy: a longitudinal pilot study. PeerJ 2020; 8: e8617
- Mohanty L, Sahoo D. Prevalence and risk factors of sarcopenia: a study in a tertiary care centre. International Journal of Advances in Medicine 2016; 3: 364-36
- Chargi N, Bril SI, Emmelot-Vonk MH, de Bree R. Sarcopenia is a prognostic factor for overall survival in elderly patients with head-and-neck cancer. Eur Arch Otorhinolaryngol 2019; 276 (05) 1475-1486
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature 2008; 454 (7203): 436-444
- Kalinkovich A, Livshits G. Sarcopenic obesity or obese sarcopenia: a cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res Rev 2017; 35: 200-221
- Lee J, Liu SH, Dai KY. et al. Sarcopenia and systemic inflammation synergistically impact survival in oral cavity cancer. Laryngoscope 2021; 131 (05) E1530-E1538
- Malmstrom TK, Miller DK, Simonsick EM, Ferrucci L, Morley JE. SARC-F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle 2016; 7 (01) 28-36
- Dodds RM, Syddall HE, Cooper R. et al. Grip strength across the life course: normative data from twelve British studies. PLoS One 2014; 9 (12) e113637
- Studenski SA, Peters KW, Alley DE. et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci 2014; 69 (05) 547-558
- Yamada Y, Nishizawa M, Uchiyama T. et al. Developing and validating an age-independent equation using multi-frequency bioelectrical impedance analysis for estimation of appendicular skeletal muscle mass and establishing a cutoff for sarcopenia. Int J Environ Res Public Health 2017; 14 (07) 809 DOI: 10.3390/ijerph14070809.
- Pavasini R, Guralnik J, Brown JC. et al. Short physical performance battery and all-cause mortality: systematic review and meta-analysis. BMC Med 2016; 14 (01) 215
- Bischoff HA, Stähelin HB, Monsch AU. et al. Identifying a cut-off point for normal mobility: a comparison of the timed ‘up and go’ test in community-dwelling and institutionalised elderly women. Age Ageing 2003; 32 (03) 315-320
- Shankaran M, Czerwieniec G, Fessler C. et al. Dilution of oral D 3 -Creatine to measure creatine pool size and estimate skeletal muscle mass: development of a correction algorithm. J Cachexia Sarcopenia Muscle 2018; 9 (03) 540-546
- Jung AR, Roh JL, Kim JS, Choi SH, Nam SY, Kim SY. The impact of skeletal muscle depletion on older adult patients with head and neck cancer undergoing primary surgery. J Geriatr Oncol 2021; 12 (01) 128-133
- van Rijn-Dekker MI, van den Bosch L, van den Hoek JGM. et al. Impact of sarcopenia on survival and late toxicity in head and neck cancer patients treated with radiotherapy. Radiother Oncol 2020; 147: 103-110
- Yoshimura T, Suzuki H, Takayama H. et al. Impact of preoperative low prognostic nutritional index and high intramuscular adipose tissue content on outcomes of patients with oral squamous cell carcinoma. Cancers (Basel) 2020; 12 (11) 385
- Takenaka Y, Oya R, Takemoto N, Inohara H. Predictive impact of sarcopenia in solid cancers treated with immune checkpoint inhibitors: a meta-analysis. J Cachexia Sarcopenia Muscle 2021; 12 (05) 1122-1135
- Nesemeier R, Dunlap N, McClave SA, Tennant P. Evidence-based support for nutrition therapy in head and neck cancer. Curr Surg Rep 2017; 5 (08) 18
- van den Berg MG, Rasmussen-Conrad EL, van Nispen L, van Binsbergen JJ, Merkx MA. A prospective study on malnutrition and quality of life in patients with head and neck cancer. Oral Oncol 2008; 44 (09) 830-837
- Cannon RB, Houlton JJ, Mendez E, Futran ND. Methods to reduce postoperative surgical site infections after head and neck oncology surgery. Lancet Oncol 2017; 18 (07) e405-e413
- Makiguchi T, Yamaguchi T, Nakamura H. et al. Impact of skeletal muscle mass volume on surgical site infection in free flap reconstruction for oral cancer. Microsurgery 2019; 39 (07) 598-604
- Lodders JN, Schulten EA, de Visscher JG, Forouzanfar T, Karagozoglu KH. Complications and risk after mandibular reconstruction with fibular free flaps in patients with oral squamous cell carcinoma: a retrospective cohort study. J Reconstr Microsurg 2016; 32 (06) 455-463
- Karsten RT, Al-Mamgani A, Bril SI. et al. Sarcopenia, a strong determinant for prolonged feeding tube dependency after chemoradiotherapy for head and neck cancer. Head Neck 2019; 41 (11) 4000-4008
- Surov A, Wienke A. Low skeletal muscle mass predicts relevant clinical outcomes in head and neck squamous cell carcinoma. A meta-analysis. Ther Adv Med Oncol 2021;13:17588359211008844
- Stone L, Olson B, Mowery A. et al. Association between sarcopenia and mortality in patients undergoing surgical excision of head and neck cancer. JAMA Otolaryngol Head Neck Surg 2019; 145 (07) 647-654
- Dandekar M, Tuljapurkar V, Dhar H, Panwar A, DCruz AK. Head and neck cancers in India. J Surg Oncol 2017; 115 (05) 555-563
- Tyrovolas S, Koyanagi A, Olaya B. et al. Factors associated with skeletal muscle mass, sarcopenia, and sarcopenic obesity in older adults: a multi-continent study. J Cachexia Sarcopenia Muscle 2016; 7 (03) 312-321
Address for correspondence
Hitesh R. Singhavi, MDS1131, OPD B, Department of Surgical Oncology, Fortis HospitalMulund, Mumbai 400078, MaharashtraIndiaEmail: hitsinx@gmail.comPublication History
Article published online:
08 May 2023© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Fig 1 : Inter-relation of sarcopenia on treatment of head and neck cancers (HNC).
References
- Vigneswaran N, Williams MD. Epidemiologic trends in head and neck cancer and aids in diagnosis. Oral Maxillofac Surg Clin North Am 2014; 26 (02) 123-141
- Kulkarni MR. Head and neck cancer burden in India. Int J Head Neck Surg 2013; 4 (01) 29-35
- The Global Cancer Observatory - March, 2021. Accessed April 18, 2023 at: https://gco.iarc.fr
- Lo Nigro C, Denaro N, Merlotti A, Merlano M. Head and neck cancer: improving outcomes with a multidisciplinary approach. Cancer Manag Res 2017; 9: 363-371
- Anand AK, Agarwal JP, D'Cruz A. et al. Evolving multidisciplinary treatment of squamous cell carcinoma of the head and neck in India ✰ . Cancer Treat Res Commun 2021; 26: 100269
- Bonner JA, Harari PM, Giralt J. et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 2006; 354 (06) 567-578
- Pignon JP, le Maître A, Maillard E, Bourhis J. MACH-NC Collaborative Group. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol 2009; 92 (01) 4-14
- Ganju RG, Morse R, Hoover A, TenNapel M, Lominska CE. The impact of sarcopenia on tolerance of radiation and outcome in patients with head and neck cancer receiving chemoradiation. Radiother Oncol 2019; 137: 117-124
- Alwani MM, Jones AJ, Novinger LJ. et al. Impact of sarcopenia on outcomes of autologous head and neck free tissue reconstruction. J Reconstr Microsurg 2020; 36 (05) 369-378
- Ansari E, Chargi N, van Gemert JTM. et al. Low skeletal muscle mass is a strong predictive factor for surgical complications and a prognostic factor in oral cancer patients undergoing mandibular reconstruction with a free fibula flap. Oral Oncol 2020; 101: 104530
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM. et al; European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on Sarcopenia in older people. Age Ageing 2010; 39 (04) 412-423
- Takenaka Y, Takemoto N, Oya R, Inohara H. Prognostic impact of sarcopenia in patients with head and neck cancer treated with surgery or radiation: a meta-analysis. PLoS One 2021; 16 (10) e0259288
- Chauhan NS, Samuel SR, Meenar N, Saxena PP, Keogh JWL. Sarcopenia in male patients with head and neck cancer receiving chemoradiotherapy: a longitudinal pilot study. PeerJ 2020; 8: e8617
- Mohanty L, Sahoo D. Prevalence and risk factors of sarcopenia: a study in a tertiary care centre. International Journal of Advances in Medicine 2016; 3: 364-36
- Chargi N, Bril SI, Emmelot-Vonk MH, de Bree R. Sarcopenia is a prognostic factor for overall survival in elderly patients with head-and-neck cancer. Eur Arch Otorhinolaryngol 2019; 276 (05) 1475-1486
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature 2008; 454 (7203): 436-444
- Kalinkovich A, Livshits G. Sarcopenic obesity or obese sarcopenia: a cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res Rev 2017; 35: 200-221
- Lee J, Liu SH, Dai KY. et al. Sarcopenia and systemic inflammation synergistically impact survival in oral cavity cancer. Laryngoscope 2021; 131 (05) E1530-E1538
- Malmstrom TK, Miller DK, Simonsick EM, Ferrucci L, Morley JE. SARC-F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle 2016; 7 (01) 28-36
- Dodds RM, Syddall HE, Cooper R. et al. Grip strength across the life course: normative data from twelve British studies. PLoS One 2014; 9 (12) e113637
- Studenski SA, Peters KW, Alley DE. et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci 2014; 69 (05) 547-558
- Yamada Y, Nishizawa M, Uchiyama T. et al. Developing and validating an age-independent equation using multi-frequency bioelectrical impedance analysis for estimation of appendicular skeletal muscle mass and establishing a cutoff for sarcopenia. Int J Environ Res Public Health 2017; 14 (07) 809 DOI: 10.3390/ijerph14070809.
- Pavasini R, Guralnik J, Brown JC. et al. Short physical performance battery and all-cause mortality: systematic review and meta-analysis. BMC Med 2016; 14 (01) 215
- Bischoff HA, Stähelin HB, Monsch AU. et al. Identifying a cut-off point for normal mobility: a comparison of the timed ‘up and go’ test in community-dwelling and institutionalised elderly women. Age Ageing 2003; 32 (03) 315-320
- Shankaran M, Czerwieniec G, Fessler C. et al. Dilution of oral D 3 -Creatine to measure creatine pool size and estimate skeletal muscle mass: development of a correction algorithm. J Cachexia Sarcopenia Muscle 2018; 9 (03) 540-546
- Jung AR, Roh JL, Kim JS, Choi SH, Nam SY, Kim SY. The impact of skeletal muscle depletion on older adult patients with head and neck cancer undergoing primary surgery. J Geriatr Oncol 2021; 12 (01) 128-133
- van Rijn-Dekker MI, van den Bosch L, van den Hoek JGM. et al. Impact of sarcopenia on survival and late toxicity in head and neck cancer patients treated with radiotherapy. Radiother Oncol 2020; 147: 103-110
- Yoshimura T, Suzuki H, Takayama H. et al. Impact of preoperative low prognostic nutritional index and high intramuscular adipose tissue content on outcomes of patients with oral squamous cell carcinoma. Cancers (Basel) 2020; 12 (11) 385
- Takenaka Y, Oya R, Takemoto N, Inohara H. Predictive impact of sarcopenia in solid cancers treated with immune checkpoint inhibitors: a meta-analysis. J Cachexia Sarcopenia Muscle 2021; 12 (05) 1122-1135
- Nesemeier R, Dunlap N, McClave SA, Tennant P. Evidence-based support for nutrition therapy in head and neck cancer. Curr Surg Rep 2017; 5 (08) 18
- van den Berg MG, Rasmussen-Conrad EL, van Nispen L, van Binsbergen JJ, Merkx MA. A prospective study on malnutrition and quality of life in patients with head and neck cancer. Oral Oncol 2008; 44 (09) 830-837
- Cannon RB, Houlton JJ, Mendez E, Futran ND. Methods to reduce postoperative surgical site infections after head and neck oncology surgery. Lancet Oncol 2017; 18 (07) e405-e413
- Makiguchi T, Yamaguchi T, Nakamura H. et al. Impact of skeletal muscle mass volume on surgical site infection in free flap reconstruction for oral cancer. Microsurgery 2019; 39 (07) 598-604
- Lodders JN, Schulten EA, de Visscher JG, Forouzanfar T, Karagozoglu KH. Complications and risk after mandibular reconstruction with fibular free flaps in patients with oral squamous cell carcinoma: a retrospective cohort study. J Reconstr Microsurg 2016; 32 (06) 455-463
- Karsten RT, Al-Mamgani A, Bril SI. et al. Sarcopenia, a strong determinant for prolonged feeding tube dependency after chemoradiotherapy for head and neck cancer. Head Neck 2019; 41 (11) 4000-4008
- Surov A, Wienke A. Low skeletal muscle mass predicts relevant clinical outcomes in head and neck squamous cell carcinoma. A meta-analysis. Ther Adv Med Oncol 2021;13:17588359211008844
- Stone L, Olson B, Mowery A. et al. Association between sarcopenia and mortality in patients undergoing surgical excision of head and neck cancer. JAMA Otolaryngol Head Neck Surg 2019; 145 (07) 647-654
- Dandekar M, Tuljapurkar V, Dhar H, Panwar A, DCruz AK. Head and neck cancers in India. J Surg Oncol 2017; 115 (05) 555-563
- Tyrovolas S, Koyanagi A, Olaya B. et al. Factors associated with skeletal muscle mass, sarcopenia, and sarcopenic obesity in older adults: a multi-continent study. J Cachexia Sarcopenia Muscle 2016; 7 (03) 312-321


 PDF
PDF  Views
Views  Share
Share

