Is there any role of mast cell density and microvessel density in cervical squamous cell carcinoma? A histologic study with special reference to CD-34 immunomarker staining
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2014; 35(02): 165-169
DOI: DOI: 10.4103/0971-5851.138994
Abstract
Background: Mast cells are involved in induction of angiogenesis in the early-stages of tumor development and in modulating blood vessel growth in the later stages of tumor progression. Aims and Objectives: This study was carried out to evaluate the association between mast cell density (MCD) and microvessel density (MVD) in carcinoma in situ (CIS), microinvasive carcinoma (CA) and invasive squamous cell CA of cervix. Materials and Methods: Six cases of CIS, four cases of microinvasive CA and 38 cases of invasive CA were studied over a period of 2 years from August, 2011 to June, 2013. Ten control samples were included in the study. Routine histologic examination was done. Toluidine blue stain was used for MCD determination. Immunohistochemical analysis with CD-34 was done for assessing MVD. Student′s t-test was used to calculate the statistical significance of MCD and MVD. Results: Both MCD and MVD increased from normal samples through CIS to invasive cervical CA. In the four cases of microinvasive CA, the MCD and MVD were more than that of the control samples, but less than that of the six cases of CIS. Conclusion: There is a correlation between mast cell accumulation and angiogenesis in CIS, microinvasive CA and invasive cervical squamous cell CA. MCD and MVD in invasive CA exceed those in CIS and microinvasive CA. It gives us an opportunity to postulate that therapeutic strategies against mast cell mediators and angiogenesis may be of benefit in patients of early-stage cervical CA.
Publication History
Article published online:
19 July 2021
© 2014. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:
Mast cells are involved in induction of angiogenesis in the early-stages of tumor development and in modulating blood vessel growth in the later stages of tumor progression.
Aims and Objectives:
This study was carried out to evaluate the association between mast cell density (MCD) and microvessel density (MVD) in carcinoma in situ (CIS), microinvasive carcinoma (CA) and invasive squamous cell CA of cervix.
Materials and Methods:
Six cases of CIS, four cases of microinvasive CA and 38 cases of invasive CA were studied over a period of 2 years from August, 2011 to June, 2013. Ten control samples were included in the study. Routine histologic examination was done. Toluidine blue stain was used for MCD determination. Immunohistochemical analysis with CD-34 was done for assessing MVD. Student's t-test was used to calculate the statistical significance of MCD and MVD.
Results:
Both MCD and MVD increased from normal samples through CIS to invasive cervical CA. In the four cases of microinvasive CA, the MCD and MVD were more than that of the control samples, but less than that of the six cases of CIS.
Conclusion:
There is a correlation between mast cell accumulation and angiogenesis in CIS, microinvasive CA and invasive cervical squamous cell CA. MCD and MVD in invasive CA exceed those in CIS and microinvasive CA. It gives us an opportunity to postulate that therapeutic strategies against mast cell mediators and angiogenesis may be of benefit in patients of early-stage cervical CA.
Mast cells have a wide variety of functions, which not only encompass regulation of immune responses, but also autoimmunity, tolerance to graft rejection, promotion of or protection from cancer, wound healing, angiogenesis, cardiovascular diseases, diabetes mellitus, obesity, and other significant diseases. In the previous days, these cells were considered as important due to their role in Ig-E driven allergic reactions. However, today the wide spectrum of properties of mast cells is well-recognized.[1]
Mast cells have been found to be associated with different types of tumors. They play an important role in the induction of angiogenesis in the early-stages of development of tumor. Further, during tumor progression they help to modulate the blood vessel growth.[2] The association of angiogenesis and cancer has been credited to the visionary pioneer Folkman (1933-2008), who first stated that tumor growing was directly dependent on the blood vessel network development.[3]
Experimentally induced tumors have shown mast cell accumulation close to the tumor cells before the onset of angiogenesis.[4] On the other hand, tumors induced in mast cell-deficient mice have shown reduced angiogenesis and metastatic potential.[5] Acikalin et al. found that microvessel density (MVD) was a significant prognostic factor in colorectal carcinoma (CA). They concluded that microvessel counts and mast cell density (MCD) have a significant correlation with one another, which prompted them to suggest that mast cells may play a role in promoting angiogenesis thereby assisting in tumor progression.[6] In their study Bochner et al. they found in their study that MVD is an important prognostic factor in case of invasive transitional cell CA of bladder.[7] Erovic et al. concluded that tumor neovascularization might play a vital role in the prognosis of patients with squamous cell CA of the head and neck.[8]
Invasive squamous cell carcinoma (SCC) of the cervix is the most common malignant tumor of the female genital tract in most countries, especially in developing countries like India and the most frequent neoplasm among women in many of them.[9] This study was done to evaluate the relationship between MCD and MVD in carcinoma in situ (CIS), microinvasive carcinoma (microinvasive CA) and invasive squamous cell cervical CA. The term CIS was employed when there was no differentiation at any level of the cervical squamous epithelium (despite some occasional flattening of the surface cells) and the basal cell was disorganized. The term CIS has now been incorporated within the domain of high grade squamous intraepithelial lesion, under the Bethesda classification. Even though this classification was originally designed for the cytologic specimens, some pathologists apply the same for histologic samples. Microscopically, three major categories of cervical SCC exist: Large cell nonkeratinizing, keratinizing, and small cell.[9]
This study was carried out over a period of 2 years from August, 2011 to June, 2013, in our department. We studied 6 cases of CIS, 4 cases of microinvasive SCC (microinvasive SCC) and 38 cases of invasive cervical CA (invasive CA). Control sections were taken from 10 normal cervical tissue samples.
All these tissue sections were obtained from patients who had undergone total abdominal hysterectomy with or without bilateral oopherectomy. Cervical punch biopsies and cases of cervical intraepithelial neoplasia (CIN) Grades 1 and 2 were excluded from our study. CIN 3/CIS, microinvasive CA and invasive cervical CA were included in the study.
The samples were fixed in 10% formalin and embedded in paraffin, according to standard procedures. 5 μm thick sections were cut and mounted on glass slides. For each case, three sections were obtained: One for routine hematoxylin and eosin staining, one for toluidine blue staining and the third for immunohistochemical analysis with CD-34. For immunohistochemistry, the sections were mounted on poly-L-lysine coated slides.
The toluidine blue stain gives a light blue background to the section with mast cells appearing red-purple in color. This allows easy mapping of stained mast cells. Ten “hot spots” (areas with the highest MCD) from each slide were identified. The total of the counts of the ten fields was recorded as the mast cell count per 10 hpf.
Initially, we used the Giemsa stain to delineate the mast cells. However, we found that the toluidine blue staining method was simpler and it produced better results.
Single endothelial cell or clusters of endothelial cells positive for CD-34 were considered as a microvessel. The presence of blood cells or fibrin without any detectable endothelial cells is not sufficient to define a microvessel. Vessels with muscular walls were not counted. Three “hot spots” (areas with the highest microvessel concentration) from each slide were identified. MVD of the sample was estimated as a mean of MVD in three histological fields and it was recorded as the MVD per hpf. All the data were recorded and analyzed using appropriate statistical tests.
RESULTS
We studied 48 cases, of which 38 were of invasive CA, 4 of microinvasive SCC and 6 of CIS. The mean age of the patients of invasive CA was 43.8 years. The mean diameter of these tumors was 4.6 cm. Among the 38 cases of invasive CA, 12 were found to be in the stage pT1b1 and 26 in the stage pT1b2. All the 4 cases of microinvasive CA were in the stage pT1a2.
Among the 38 cases of invasive cervical CA, 34 cases were of large cell nonkeratinizing type. Clear cell differentiation was present in 4 of these 34 cases. Four cases were of large cell keratinizing type [Figure 1].
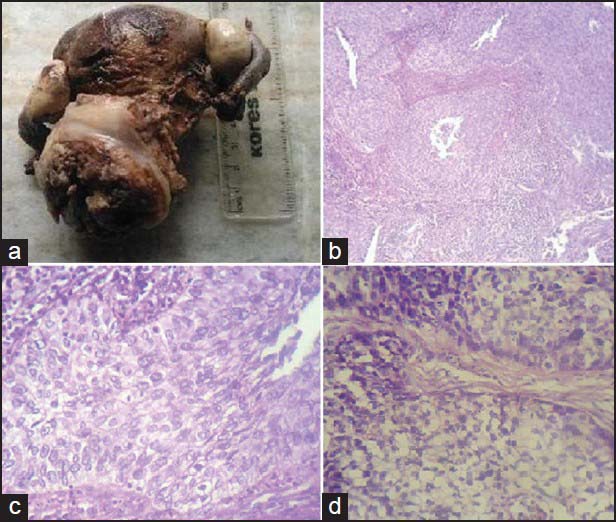
| Figure 1:(a) Gross appearance of a case of invasive cervical carcinoma; (b) microphotograph showing nonkeratinizing invasive squamous cell carcinoma (SCC) of cervix, ×100; (c) microphotograph showing nonkeratinizing invasive SCC of cervix, ×400; (d) microphotograph showing nonkeratinizing invasive SCC of cervix with clear cell differentiation, ×400
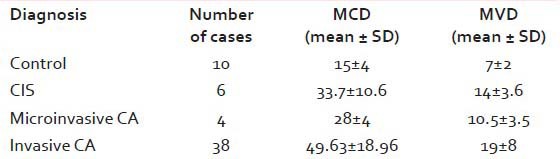
We found that both MCD and MVD increased from normal samples through CIS to invasive cervical CA [Figures [Figures22 and and3].3]. In the four cases of microinvasive CA, the MCD and MVD were more than that of the control samples but less than that of the six cases of CIS [Table 1].
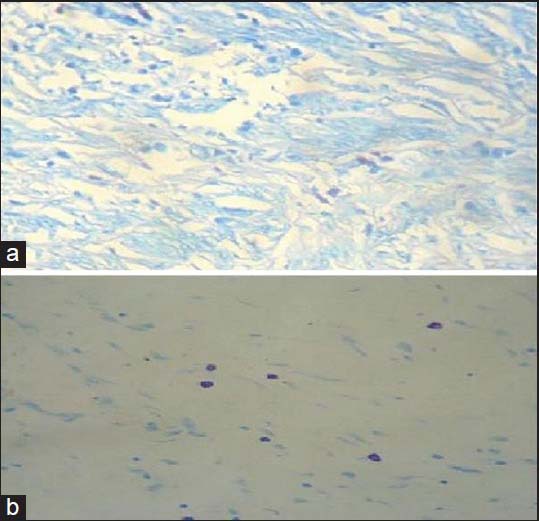
| Figure 2:Microphotograph showing mast cells in invasive cervical squamous cell carcinoma; (a) Giemsa stain, ×400; (b) toluidine blue stain, ×400
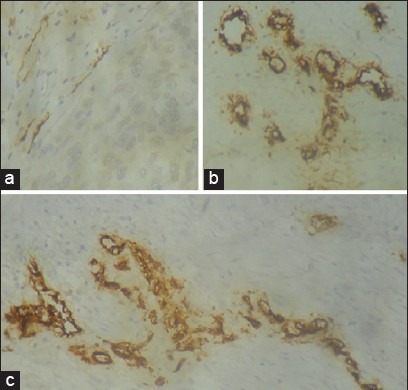
| Figure 3:Microphotograph showing microvessels by CD-34 staining in (a) control cervical tissue samples, ×400; (b) microinvasive carcinoma of cervix, ×400; (c) invasive cervical squamous cell carcinoma of cervix, ×400
Table 1
MCD and MVD in control samples, CIS, microinvasive CA and invasive CA
|
The mast cells were mostly found located at the interface between the tumor tissue and underlying stroma. Many of them were located around the microvessels.
Statistical significance of MCD and MVD as determined by Student's t-test is shown in Tables Tables22 and and33
Table 2
Statistical significance of MCD when compared by student's t-test

|
Table 3
Statistical significance of MVD when compared by student's t-test

|
DISCUSSION
The presence of mast cells in tumor tissue was first reported by Ehrlich in 1878.[10] The role of mast cells in tumor formation and progression is complex. Current studies show both positive and negative relationships between MCD and tumor progression.[11,12,13,14,15] Mast cells were thought to play a pivotal role in angiogenesis due to their close association with blood vessels and lymphatic channels. These cells were also found to accumulate in substantial numbers in richly vascularized tissues such as hemangiomas, polyps, and tumors.[16]
The data on relationship between MCD and MVD in cervical cancers and pretumoral conditions are scanty.[17] In our study, we found that both MCD and MVD increased from normal cervical samples through CIS to invasive CA. But, in the four cases of microinvasive SCC in our study, both MCD and MVD were higher than that of the control samples but less than that of CIS. We found highest MCD (102/10 hpf) in a case of large cell nonkeratinizing SCC with clear cell differentiation (stage pT1b2). Lowest MCD (20/10 hpf) was found in two cases of large cell nonkeratinizing SCC (stage pT1b1). In our study, lowest MVD (2/hpf) was found in a case of large cell nonkeratinizing SCC (stage pT1b1). We found highest MVD (35/hpf) in another case of large cell nonkeratinizing SCC (stage pT1b2).
Cabanillas-Saez et al. reported that the MCD remained constant through the Grades 1-3 of CIN, but it significantly increased in invasive cervical CA. They concluded that the mast cells provide an effective mechanism to create the vascularized microenvironment necessary for tumor cells to proliferate and disseminate.[18] Utrera-Barillas et al. proved by their results that mast cells and macrophages are of vital importance in the development of tumor associated blood and lymphatic capillaries in cervical CA.[19] Benítez-Bribiesca et al. found that the MVD in normal epithelium and in dysplasias was similar but they observed a significant increase in CIS and invasive cervical CA.[20] Sotiropoulou et al. found an increase in MVD from the control samples through CIS to microinvasive cervical CA.[21] Wilk et al. found similar increase both in MCD and MVD in their study.[17]
On the other hand, Naik et al. reported that mast cells increase in inflammatory conditions of cervix, but decrease in malignant conditions.[22] Similar findings were also noted by Jain et al.[23]
Vieira et al. compared 3 endothelial markers, antiCD-31, antiCD-34 and BNH9, for evaluation of angiogenesis in cervical cancers. They concluded that antiCD-34 and BNH9 have higher sensitivity than antiCD-31.[24] In this study, antiCD-34 has been used for assessment of MVD.
In our study, all the 38 cases of invasive cervical CA belonged to stage pT1. This is probably because surgical treatment or hysterectomy is considered only for early-stage cervical CA. We also noted that during our study period of 2 years, we obtained a large number of cases of invasive cervical CA (38), compared to the number of cases of CIS (6) and microinvasive CA (4). We attributed this discrepancy with the poor socioeconomic conditions and lack of awareness among the patients of developing countries like ours. The patients fail to seek medical help at an early-stage of the disease, thus contributing to the large number of cases of invasive CA that we encountered.
Several studies have found a correlation between MVD and prognosis in cases of cervical cancers. Our study is limited by the fact that a similar assessment could not be done, since we lost most of our patients to follow-up. Randall et al. concluded in their study that MVD is an important prognostic factor in high-risk early-stage cervical cancer.[25] Ancuta et al. and Lenczewski et al. independently reported in their studies that higher MVD is associated with poor overall survival rates.[26,27] Tjalma et al. found similar significance of MVD and they suggested that in future, this criterion may be used for selection of patients for antiangiogenesis therapy.[28]
A correlation between MCD and tumor progression has been reported in malignancies of other organs as well. Mukherjee et al. found that MCD was higher in well-differentiated gastric cancers than in control subjects. They also found that poorly-differentiated gastric cancers had lower MCD than well-differentiated ones.[29] Ribatti et al. reported in their study that MCD is correlated with angiogenesis and progression of tumor in patients with gastric CA.[30] Elezoglu and Tolunay et al. found that there is an association between MCD and MVD in colorectal CA. They reported that the grade of colorectal CA increased with the number of mast cells, while survival decreased with an increase in MCD.[31] In our study, we found that both MCD and MVD are higher in invasive cervical CA than in CIS or microinvasive SCC.
CONCLUSION
Mast cell accumulation is related with angiogenesis in CIS, microinvasive CA and invasive cervical CA. The values of MCD and MVD are higher in invasive cervical CAs when compared to those in CIS, microinvasive CA and control samples. Therapeutic strategies against mast cell mediators or angiogenesis may be of help in control of progression of early-stage cervical CA. However, further studies with larger sample size are required before drawing any definite conclusion.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- Rodewald HR, Feyerabend TB. Widespread immunological functions of mast cells: Fact or fiction? Immunity 2012;37:13-24.
- de Souza DA Jr, Toso VD, Campos MR, Lara VS, Oliver C, Jamur MC. Expression of mast cell proteases correlates with mast cell maturation and angiogenesis during tumor progression. PLoS One 2012;7:e40790.
- Folkman J, Merler E, Abernathy C, Williams G. Isolation of a tumor factor responsible for angiogenesis. J Exp Med 1971;133:275-88.
- Kessler DA, Langer RS, Pless NA, Folkman J. Mast cells and tumor angiogenesis. Int J Cancer 1976;18:703-9.
- Starkey JR, Crowle PK, Taubenberger S. Mast-cell-deficient W/Wv mice exhibit a decreased rate of tumor angiogenesis. Int J Cancer 1988;42:48-52.
- Acikalin MF, Oner U, Topçu I, Yasar B, Kiper H, Colak E. Tumour angiogenesis and mast cell density in the prognostic assessment of colorectal carcinomas. Dig Liver Dis 2005;37:162-9.
- Bochner BH, Cote RJ, Weidner N, Groshen S, Chen SC, Skinner DG, et al. Angiogenesis in bladder cancer: Relationship between microvessel density and tumor prognosis. J Natl Cancer Inst 1995;87:1603-12.
- Erovic BM, Neuchrist C, Berger U, El-Rabadi K, Burian M. Quantitation of microvessel density in squamous cell carcinoma of the head and neck by computer-aided image analysis. Wien Klin Wochenschr 2005;117:53-7.
- Rosai J, Ackerman LV. Uterus, cervix. In: Rosai J, Ackerman LV, editors. Rosai and Ackerman′s Surgical Pathology. 10 th ed. New Delhi: Elsevier; 2011. p. 1436-76.
- Ehrlich P. Contributions to the theory and practice of histological staining (Inaugural dissertation, University of Leipzig, 1878). In: Himmelweit F, editor. Collected Papers of Paul Ehrlich. Vol. 1. London: Pergamon Press; 1956. p. 65-98.
- Galinsky DS, Nechushtan H. Mast cells and cancer - no longer just basic science. Crit Rev Oncol Hematol 2008;68:115-30.
- Maltby S, Khazaie K, McNagny KM. Mast cells in tumor growth: Angiogenesis, tissue remodelling and immune-modulation. Biochim Biophys Acta 2009;1796:19-26.
- Moon TC, St Laurent CD, Morris KE, Marcet C, Yoshimura T, Sekar Y, et al. Advances in mast cell biology: New understanding of heterogeneity and function. Mucosal Immunol 2010;3:111-28.
- Theoharides TC, Conti P. Mast cells: The Jekyll and Hyde of tumor growth. Trends Immunol 2004;25:235-41.
- Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nat Immunol 2008;9:1215-23.
- Gilfillan AM, Beaven MA. Regulation of mast cell responses in health and disease. Crit Rev Immunol 2011;31:475-529.
- Wilk M, Liszka L, Palen P, Gabriel A, Laudanski P. Intensity of angiogenesis and mast cell infiltration in cervical intraepithelial and invasive lesions - are they correlated? Pathol Res Pract 2010;206:217-22.
- Cabanillas-Saez A, Schalper JA, Nicovani SM, Rudolph MI. Characterization of mast cells according to their content of tryptase and chymase in normal and neoplastic human uterine cervix. Int J Gynecol Cancer 2002;12:92-8.
- Utrera-Barillas D, Castro-Manrreza M, Castellanos E, Gutiérrez-Rodríguez M, Arciniega-Ruíz de Esparza O, García-Cebada J, et al. The role of macrophages and mast cells in lymphangiogenesis and angiogenesis in cervical carcinogenesis. Exp Mol Pathol 2010;89:190-6.
- Benítez-Bribiesca L, Wong A, Utrera D, Castellanos E. The role of mast cell tryptase in neoangiogenesis of premalignant and malignant lesions of the uterine cervix. J Histochem Cytochem 2001;49:1061-2.
- Sotiropoulou M, Diakomanolis E, Elsheikh A, Loutradis D, Markaki S, Michalas S. Angiogenic properties of carcinoma in situ and microinvasive carcinoma of the uterine cervix. Eur J Gynaecol Oncol 2004;25:219-21.
- Naik R, Pai MR, Poornima Baliga B, Nayak KS, Shankarnarayana, Dighe P. Mast cell profile in uterine cervix. Indian J Pathol Microbiol 2004;47:178-80.
- Jain PC, Singh SN, Pratap VK, Lahiri B. Connective tissue changes and mast cell variations in benign and malignant lesions of the uterine cervix. Int Surg 1977;62:358-60.
- Vieira SC, Zeferino LC, Da Silva BB, Aparecida Pinto G, Vassallo J, Carasan GA, et al. Quantification of angiogenesis in cervical cancer: A comparison among three endothelial cell markers. Gynecol Oncol 2004;93:121-4.
- Randall LM, Monk BJ, Darcy KM, Tian C, Burger RA, Liao SY, et al. Markers of angiogenesis in high-risk, early-stage cervical cancer: A Gynecologic Oncology Group study. Gynecol Oncol 2009;112:583-9.
- Ancuta C, Ancuta E, Zugun-Eloae F, Carasevici E. Neoangiogenesis in cervical cancer: Focus on CD34 assessment. Rom J Morphol Embryol 2010;51:289-94.
- ;Lenczewski A, Terlikowski SJ, Sulkowska M, Famulski W, Sulkowski S, Kulikowski M. Prognostic significance of CD34 expression in early cervical squamous cell carcinoma. Folia Histochem Cytobiol 2002;40:205-6.
- Tjalma W, Van Marck E, Weyler J, Dirix L, Van Daele A, Goovaerts G, et al. Quantification and prognostic relevance of angiogenic parameters in invasive cervical cancer. Br J Cancer 1998;78:170-4.
- Mukherjee S, Bandyopadhyay G, Dutta C, Bhattacharya A, Karmakar R, Barui G. Evaluation of endoscopic biopsy in gastric lesions with a special reference to the significance of mast cell density. Indian J Pathol Microbiol 2009;52:20-4.
- Ribatti D, Guidolin D, Marzullo A, Nico B, Annese T, Benagiano V, et al. Mast cells and angiogenesis in gastric carcinoma. Int J Exp Pathol 2010;91:350-6.
- Elezoglu B, Tolunay S. The relationship between the stromal mast cell number, microvessel density, c-erbB-2 staining and survival and prognostic factors in colorectal carcinoma. Turk Patoloji Derg 2012;28:110-8.

| Figure 1:(a) Gross appearance of a case of invasive cervical carcinoma; (b) microphotograph showing nonkeratinizing invasive squamous cell carcinoma (SCC) of cervix, ×100; (c) microphotograph showing nonkeratinizing invasive SCC of cervix, ×400; (d) microphotograph showing nonkeratinizing invasive SCC of cervix with clear cell differentiation, ×400

| Figure 2:Microphotograph showing mast cells in invasive cervical squamous cell carcinoma; (a) Giemsa stain, ×400; (b) toluidine blue stain, ×400

| Figure 3:Microphotograph showing microvessels by CD-34 staining in (a) control cervical tissue samples, ×400; (b) microinvasive carcinoma of cervix, ×400; (c) invasive cervical squamous cell carcinoma of cervix, ×400
References
- Rodewald HR, Feyerabend TB. Widespread immunological functions of mast cells: Fact or fiction? Immunity 2012;37:13-24.
- de Souza DA Jr, Toso VD, Campos MR, Lara VS, Oliver C, Jamur MC. Expression of mast cell proteases correlates with mast cell maturation and angiogenesis during tumor progression. PLoS One 2012;7:e40790.
- Folkman J, Merler E, Abernathy C, Williams G. Isolation of a tumor factor responsible for angiogenesis. J Exp Med 1971;133:275-88.
- Kessler DA, Langer RS, Pless NA, Folkman J. Mast cells and tumor angiogenesis. Int J Cancer 1976;18:703-9.
- Starkey JR, Crowle PK, Taubenberger S. Mast-cell-deficient W/Wv mice exhibit a decreased rate of tumor angiogenesis. Int J Cancer 1988;42:48-52.
- Acikalin MF, Oner U, Topçu I, Yasar B, Kiper H, Colak E. Tumour angiogenesis and mast cell density in the prognostic assessment of colorectal carcinomas. Dig Liver Dis 2005;37:162-9.
- Bochner BH, Cote RJ, Weidner N, Groshen S, Chen SC, Skinner DG, et al. Angiogenesis in bladder cancer: Relationship between microvessel density and tumor prognosis. J Natl Cancer Inst 1995;87:1603-12.
- Erovic BM, Neuchrist C, Berger U, El-Rabadi K, Burian M. Quantitation of microvessel density in squamous cell carcinoma of the head and neck by computer-aided image analysis. Wien Klin Wochenschr 2005;117:53-7.
- Rosai J, Ackerman LV. Uterus, cervix. In: Rosai J, Ackerman LV, editors. Rosai and Ackerman′s Surgical Pathology. 10 th ed. New Delhi: Elsevier; 2011. p. 1436-76.
- Ehrlich P. Contributions to the theory and practice of histological staining (Inaugural dissertation, University of Leipzig, 1878). In: Himmelweit F, editor. Collected Papers of Paul Ehrlich. Vol. 1. London: Pergamon Press; 1956. p. 65-98.
- Galinsky DS, Nechushtan H. Mast cells and cancer - no longer just basic science. Crit Rev Oncol Hematol 2008;68:115-30.
- Maltby S, Khazaie K, McNagny KM. Mast cells in tumor growth: Angiogenesis, tissue remodelling and immune-modulation. Biochim Biophys Acta 2009;1796:19-26.
- Moon TC, St Laurent CD, Morris KE, Marcet C, Yoshimura T, Sekar Y, et al. Advances in mast cell biology: New understanding of heterogeneity and function. Mucosal Immunol 2010;3:111-28.
- Theoharides TC, Conti P. Mast cells: The Jekyll and Hyde of tumor growth. Trends Immunol 2004;25:235-41.
- Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nat Immunol 2008;9:1215-23.
- Gilfillan AM, Beaven MA. Regulation of mast cell responses in health and disease. Crit Rev Immunol 2011;31:475-529.
- Wilk M, Liszka L, Palen P, Gabriel A, Laudanski P. Intensity of angiogenesis and mast cell infiltration in cervical intraepithelial and invasive lesions - are they correlated? Pathol Res Pract 2010;206:217-22.
- Cabanillas-Saez A, Schalper JA, Nicovani SM, Rudolph MI. Characterization of mast cells according to their content of tryptase and chymase in normal and neoplastic human uterine cervix. Int J Gynecol Cancer 2002;12:92-8.
- Utrera-Barillas D, Castro-Manrreza M, Castellanos E, Gutiérrez-Rodríguez M, Arciniega-Ruíz de Esparza O, García-Cebada J, et al. The role of macrophages and mast cells in lymphangiogenesis and angiogenesis in cervical carcinogenesis. Exp Mol Pathol 2010;89:190-6.
- Benítez-Bribiesca L, Wong A, Utrera D, Castellanos E. The role of mast cell tryptase in neoangiogenesis of premalignant and malignant lesions of the uterine cervix. J Histochem Cytochem 2001;49:1061-2.
- Sotiropoulou M, Diakomanolis E, Elsheikh A, Loutradis D, Markaki S, Michalas S. Angiogenic properties of carcinoma in situ and microinvasive carcinoma of the uterine cervix. Eur J Gynaecol Oncol 2004;25:219-21.
- Naik R, Pai MR, Poornima Baliga B, Nayak KS, Shankarnarayana, Dighe P. Mast cell profile in uterine cervix. Indian J Pathol Microbiol 2004;47:178-80.
- Jain PC, Singh SN, Pratap VK, Lahiri B. Connective tissue changes and mast cell variations in benign and malignant lesions of the uterine cervix. Int Surg 1977;62:358-60.
- Vieira SC, Zeferino LC, Da Silva BB, Aparecida Pinto G, Vassallo J, Carasan GA, et al. Quantification of angiogenesis in cervical cancer: A comparison among three endothelial cell markers. Gynecol Oncol 2004;93:121-4.
- Randall LM, Monk BJ, Darcy KM, Tian C, Burger RA, Liao SY, et al. Markers of angiogenesis in high-risk, early-stage cervical cancer: A Gynecologic Oncology Group study. Gynecol Oncol 2009;112:583-9.
- Ancuta C, Ancuta E, Zugun-Eloae F, Carasevici E. Neoangiogenesis in cervical cancer: Focus on CD34 assessment. Rom J Morphol Embryol 2010;51:289-94.
- ;Lenczewski A, Terlikowski SJ, Sulkowska M, Famulski W, Sulkowski S, Kulikowski M. Prognostic significance of CD34 expression in early cervical squamous cell carcinoma. Folia Histochem Cytobiol 2002;40:205-6.
- Tjalma W, Van Marck E, Weyler J, Dirix L, Van Daele A, Goovaerts G, et al. Quantification and prognostic relevance of angiogenic parameters in invasive cervical cancer. Br J Cancer 1998;78:170-4.
- Mukherjee S, Bandyopadhyay G, Dutta C, Bhattacharya A, Karmakar R, Barui G. Evaluation of endoscopic biopsy in gastric lesions with a special reference to the significance of mast cell density. Indian J Pathol Microbiol 2009;52:20-4.
- Ribatti D, Guidolin D, Marzullo A, Nico B, Annese T, Benagiano V, et al. Mast cells and angiogenesis in gastric carcinoma. Int J Exp Pathol 2010;91:350-6.
- Elezoglu B, Tolunay S. The relationship between the stromal mast cell number, microvessel density, c-erbB-2 staining and survival and prognostic factors in colorectal carcinoma. Turk Patoloji Derg 2012;28:110-8.


 PDF
PDF  Views
Views  Share
Share

