Kinase domain mutations and responses to dose escalation in chronic myeloid leukemia resistant to standard dose imatinib mesylate
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2013; 34(03): 221-223
DOI: DOI: 10.4103/0971-5851.123750
Abstract
Imatinib has shown unprecendeted success in the treatment of chronic myeloid leukemia (CML). However, over few years there have been reports regarding the primary and secondary resistance to Imatinib dampening the overall outcome in CML patients. In this study we have tried to assess the effect of dose escalation in patients resistant to standard dose of Imatinib and correlate it with presence of ABL kinase domain (KD) mutations. There were 90 patients resistant to imatinib, out which 29 patients were identified with KD mutations. The most common mutation was T315I , 9 out of 29 patients had it. 35 (38%) responded to dose escalation and had 67% event free survival (EFS) at estimated 2 years. Our results showed that dose escalation can over come resistance in some patients especially those in cytogenetic failure.
Publication History
Article published online:
19 July 2021
© 2013. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Imatinib has shown unprecendeted success in the treatment of chronic myeloid leukemia (CML). However, over few years there have been reports regarding the primary and secondary resistance to Imatinib dampening the overall outcome in CML patients. In this study we have tried to assess the effect of dose escalation in patients resistant to standard dose of Imatinib and correlate it with presence of ABL kinase domain (KD) mutations. There were 90 patients resistant to imatinib, out which 29 patients were identified with KD mutations. The most common mutation was T315I , 9 out of 29 patients had it. 35 (38%) responded to dose escalation and had 67% event free survival (EFS) at estimated 2 years. Our results showed that dose escalation can over come resistance in some patients especially those in cytogenetic failure.
INTRODUCTION
The discovery of imatinib mesylate (IM), a BCR ABL tyrosine kinase inhibitor (TKI) has dramatically altered the natural history of chronic myeloid leukemia (CML).[1] When the ELN and NCCN guidelines to monitor disease are applied, a third of all chronic phase CML (CP CML) patients are likely to be categorized as resistant to IM 400 mg.[2] Resistance can result from BCR ABL dependent mechanisms through mutations in the ABL kinase domain (KD) or those independent of the BCR ABL domain.[3]
For patients with resistance, IM dose escalation, 2nd generation TKIs (Dasatinib and Nilotinib), allo geneic stem cell transplantation SCT are options. There is conflicting data on the efficacy of dose escalation in patients resistant to IM 400 mg.[4,5,6] Due to financial reasons IM dose escalation remains the only option for most patients in our country. IM resistance mutations can help make a rational decision for patients with resistance. This analysis aimed at analyzing KD mutations and response to dose escalation for patients in CP-CML at resistance to standard dose IM.
PATIENTS AND METHOD
All the patients who had loss of response or no response to imatinib were included in the study. All these patients underwent imatinib resistant mutation analysis (IRMA) and dose escalation of imatinib from 400mg to 800mg once a day. These patients were then followed up and their response and survival analysis was done.
RESULTS
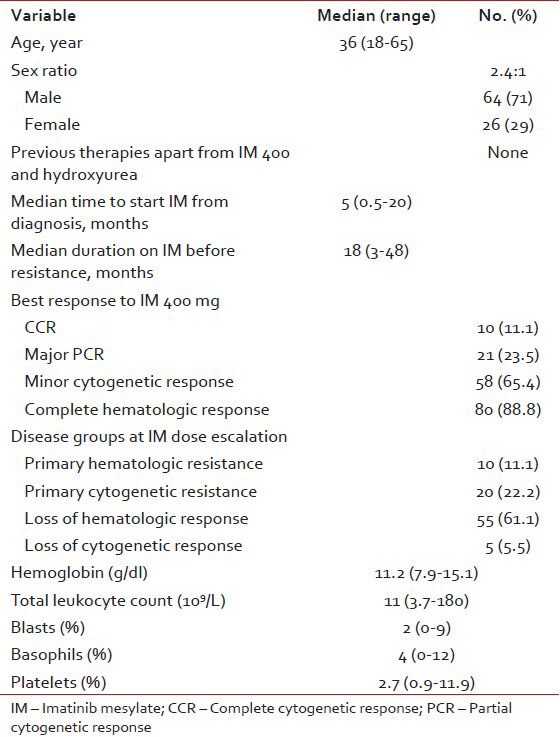
There were 90 patients with a median age of 36 years (range, 18-65). The baseline characteristics of all patients is summarized in Table 1.
Table 1
Base line characteristics (n = 90)
Twenty-nine (32.2%) patients had detectable KD mutations. These mutations are shown in Figure 1. The most common mutation was T315I in 9 (31%) patients. Thirteen (12.2%) of all patients (38% of all mutations) had a P loop mutation. N374Y is a novel mutation has not been reported before. No clinical or laboratory factors predicted for detection of KD mutation.
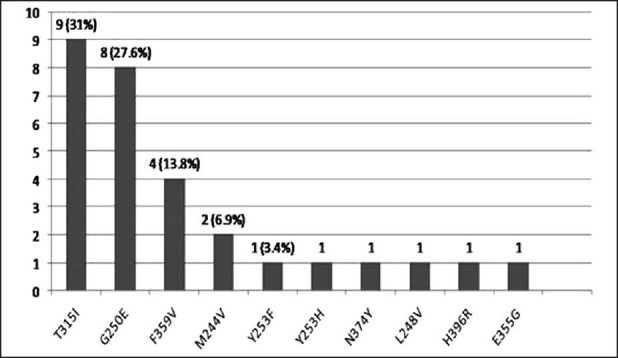
| Fig. 1 Mutations detected, n = 29 (32.2%)
Response to dose escalation
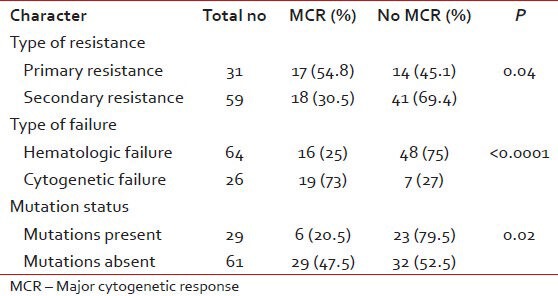
All patients with resistance were escalated from 400 mg to 800 mg. The response to dose escalation is in Table 2. 90 patients were followed-up for response, event free survival (EFS), transformation-free survival (TFS) and overall survival (OS). The median time to cytogenetic response was 11 months (range, 6-18). Predictors for achieving a major cytogenetic response (MCR) have been shown in Table 3.
Table 2
Response to dose escalation
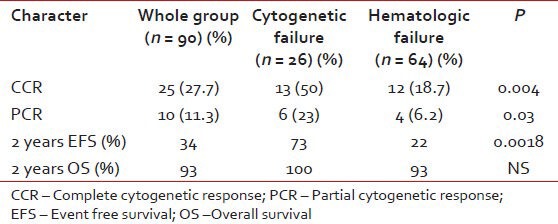
Table 3
Predictors of major cytogenetic response
Event free, transformation free and OS
At a median follow-up of 18 months (range, 3-40), 35 patients (39%) were event free and 84 patients (93%) are alive. The estimated 2 year EFS and TFS were 34% and 86%. The following are the events in 55 patients (61%): failure to achieve complete haematological response: 37 (67.2%), loss of hematologic response: 2 (3.6%), failure to achieve cytogenetic response: 7 (12.7%), progression to accelerated phase/blast crises: 3 (5.4%) and deaths: 6 (11%). Predictors of EFS are summarized in Table 4.
Table 4
Predictors of EFS
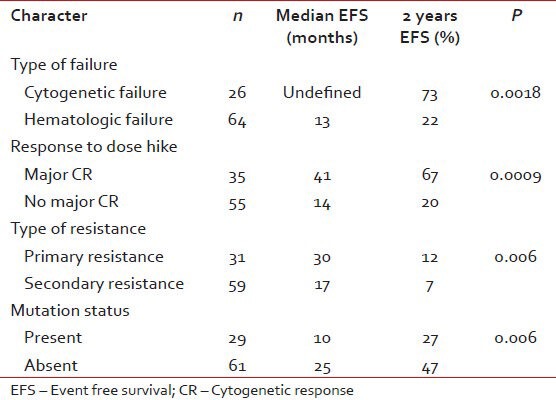
For patients with hematologic failure, the estimated 2-year EFS and TFS were 22 and 74% respectively. For those with cytogenetic failure, the 2-year EFS and TFS were 73 and 95% respectively. This EFS difference was significant, P = 0.0018. The median EFS for those who achieved MCR to dose escalation have not been reached and the projected 2 year EFS is 67%. Patients with cytogenetic failure who achieved a MCR had more sustained responses compared to those with hematologic failures with MCR, median EFS not reached versus 31 months (2 year EFS 90 vs.51%, P = 0.0006).
Adverse events
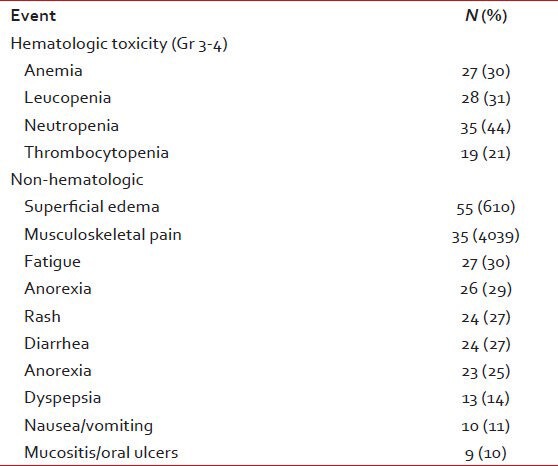
The adverse events are summarized in Table 5. Dose decreases were necessary in 16 (18%) and dose interruptions in 31 (34%). Three patients (3%) permanently discontinued IM due to adverse events. All interruptions and reductions were secondary to hematologic toxicities. 60 patients (76%) were able to continue IM at a dose more than 600 mg.
Table 5
Adverse events (n = 90)
SUMMARY AND DISCUSSION
In this analysis 32.2% of patients in CP at diagnosis of resistance to IM had a KD mutation with P loop mutations being more common. Unlike in previous studies no clinical or lab parameters were predictive of mutation detection.[7,8] T315I was much higher (31% of all mutations and 10% of all patients)than previously reported.
Our results with dose escalation are more encouraging than previously reported. However, for those resistant to standard dose IM, Dasatinib is superior to IM dose escalation in terms of cytogenetic responses and disease free survival.
The responses and EFS to IM dose escalation in our study were inferior to that reported previously. We attribute the lower MCR rate in our study to the higher percentage of patients being in hematologic failure at the time of dose escalation. In previously reported studies, patients with hematologic failures at dose escalation had poorer responses and time to treatment failure compared to those with cytogenetic failures.
Presence of mutations predicted for poorer responses and EFS to dose escalation. There is conflicting data on the prognostic value of mutations. IM dose escalation is likely to be effective only in those harboring no or relatively sensitive KD mutations.
The type of failure at dose escalation and achievement of MCR to dose escalation predicted for improved EFS. Patients with hematologic failure are less likely to have sustained MCRs.
Our results suggest that IM dose escalation is a useful strategy for those with cytogenetic failures. Those with hematologic failures are less likely to benefit from dose escalation. These results emphasize the importance of proper monitoring to detect failures early leading to timely intervention and improved outcomes.
ACKNOWLEDGMENT
We wish to acknowledge Dabur OncQuest Laboratories, New Delhi where Imatinib resistance mutation analysis were performed.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES

| Fig. 1 Mutations detected, n = 29 (32.2%)


 PDF
PDF  Views
Views  Share
Share

