Multiple Complications Secondary to L-asparaginase In a Child with Philadelphia-Chromosome-Positive Acute Lymphoblastic Leukemia: Case Report with Review of Literature
CC BY-NC-ND 4.0 ? Indian J Med Paediatr Oncol
DOI: DOI: 10.1055/s-0042-1742615
Abstract
Even though L-asparaginase remains an essential drug for the treatment of childhood acute lymphoblastic leukemia (ALL), its use is associated with several unique toxicities. In this care report, we discuss a young boy with ALL who developed multiple complications simultaneously, including pancreatitis, gastrointestinal perforation, and left ventricular thrombus secondary to L-asparaginase during induction chemotherapy. Patient received immediate surgical intervention for the perforation and was commenced on anticoagulation therapy for the thrombus but eventually expired. This report highlights the importance of being aware of toxicities secondary to the use of L-asparaginase. Multiple complications secondary to L-asparaginase have been rarely reported previously and can be fatal.
Keywords
Philadelphia-chromosome-positive acute lymphoblastic leukemia - acute pancreatitis - ventricular thrombus - gastric perforation - L-asparaginaseSource of Support
None.
Declaration of Patient Consent
The authors certify that they have obtained all appropriate patient consent forms.
Publication History
21 April 2022 (online)
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Even though L-asparaginase remains an essential drug for the treatment of childhood acute lymphoblastic leukemia (ALL), its use is associated with several unique toxicities. In this care report, we discuss a young boy with ALL who developed multiple complications simultaneously, including pancreatitis, gastrointestinal perforation, and left ventricular thrombus secondary to L-asparaginase during induction chemotherapy. Patient received immediate surgical intervention for the perforation and was commenced on anticoagulation therapy for the thrombus but eventually expired. This report highlights the importance of being aware of toxicities secondary to the use of L-asparaginase. Multiple complications secondary to L-asparaginase have been rarely reported previously and can be fatal.
Keywords
Philadelphia-chromosome-positive acute lymphoblastic leukemia - acute pancreatitis - ventricular thrombus - gastric perforation - L-asparaginase
Introduction
L-asparaginase has become an integral part in the management of childhood acute lymphoblastic leukemia (ALL) and failure to receive its intended course has been associated with poor outcome.[1] [2] However, L-asparaginase is also associated with a number of unique toxicities, some of which can have life threatening consequences.[3] Here we present a patient with Philadelphia-Chromosome-positive Acute Lymphoblastic Leukemia (Ph+?ALL) who experienced two rare complications during induction therapy: gastric perforation and a left ventricular thrombus which led to his demise.
Case Report
An 8-year-old male presented with on and off fever, bruising over shin and chest and easy fatigability for 2 weeks. A complete blood count showed a total leukocyte count (TLC) of 153???109?/L with a differential count of 60% lymphocytes, 3% neutrophils, 0.1% eosinophils, and 27% blasts in the peripheral blood. A bone marrow examination was done, flow cytometric analysis along with FISH study which was positive for t(9;22), confirmed the diagnosis of B-lineage Ph+?ALL. A real-time quantitative polymerase chain reaction (RT-PCR) was suggestive of p190 BCR/ABL fusion transcript. Cerebrospinal fluid analysis was uninvolved for disease. He was treated as per the modified COG AALL1131 protocol. Induction chemotherapy consisted of prednisolone (60?mg/m2/day; 1?28 days), vincristine (1.5?mg/m2/d; days 1, 8, 15, and 22), native?Escherichia coli?L-asparaginase (10,000 U/m2/d; days 1,4, 7, 10, 13, 16, 19, and 22) daunorubicin (30?mg/m2/d; days 1 and 15), intrathecal methotrexate (12?mg; days 1, 8, and 30) along with daily imatinib (340?mg/m2/once daily) from day 10. Chemotherapy was administered through peripheral intravenous lines on an outpatient basis. Treatment was uneventful up to day 29 of induction when he presented with abdominal pain and fever. On examination, heart rate was 86/min, blood pressure was 100/70?mm Hg and respiration was 22/min. Abdomen was mildly distended, diffusely tender, and bowel sounds were present. He was started on intravenous fluids, intravenous antibiotics (cefoperazone-sulbactam and amikacin), and analgesics for abdominal pain. The complete blood count showed a hemoglobin of 5?g/dL, platelet of 49???109/L, and TLC of 0.42???109/L with an absolute neutrophil count of 0.1???109/L. Blood tests showed an elevated lipase (1,164 U/L), elevated D-dimer (3,700?ng/mL), with a normal serum sodium (137?mEq/L) and potassium (4.2?mEql/L) and no organism was isolated from blood culture. In view of the above symptoms in a neutropenic child, computerized tomography (CT) scan of the abdomen with contrast was performed on the day of admission which revealed a bulky pancreas with fat stranding consistent with acute pancreatitis, as well as perforation of the greater curvature of the stomach resulting in pneumoperitoneum ([Fig. 1a] and [b]). An incidental finding on CT scan was a well-defined hypodense mass in the left ventricle (LV) of the heart which an ultrasound study showed lacked vascularity; two-dimensional echocardiography confirmed a mass of 3.01???1.49?cm arising from the interventricular septum with a normal ejection fraction of 60% ([Fig. 1a] and [c]). Imaging findings and elevated D-dimer both strongly suggested a diagnosis of intraventricular thrombus. The child was shifted to the intensive care unit (ICU) where he was continued on analgesics and intravenous antibiotics and kept nil by mouth. He underwent an emergency laparotomy for his abdominal emergency. Intraoperatively, there was a single perforation on the posterior wall of the stomach, and two impending perforations on the proximal jejunal wall, all of which were closed in two layers, using 4-0 polydioxanone suture ([Fig. 1d] and [e]). The surgical procedure was uneventful. Postoperatively, the child continued to be neutropenic (absolute neutrophil count: of 0.1???109/L) with a platelet count of 40???109/L. He was continued on intravenous antibiotics and was started on the low-molecular weight heparin (LMWH) enoxaparin at 1?mg/kg/dose twice a day for the large cardiac thrombus. Given his postoperative state and neutropenia, it was decided to defer any major cardiac surgery. The day after surgery the child was extubated from the ventilator and started on clear liquids, his pancreatic enzymes had returned to normal. On the subsequent day (postoperative day 2) he developed a sudden cardiac arrest and could not be revived. Permission for autopsy was not obtained.
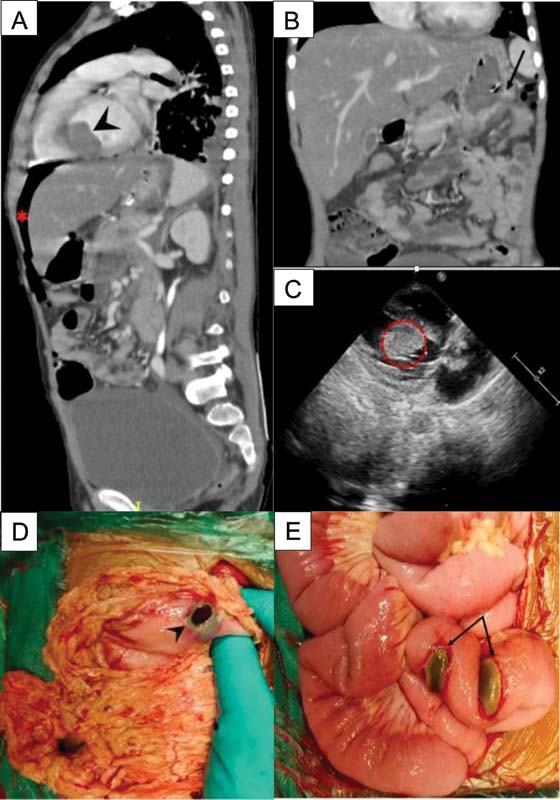
|?Fig. 1(A)CT abdomen showing left ventricular thrombus (arrow head) and pneumoperitoneum (*) (B) with gastric perforation (arrow). (C) 2D-echocardiography confirming the presence of left ventricular thrombus (outlined circle). (D) Intraoperative findings showing perforation of greater curvature of stomach (arrow head) (E) impending perforation of jejunum (double arrow).
Discussion
Philadelphia chromosome (Ph+) ALL comprises only 3%?5% of childhood ALL, the outcomes of which have been dismal until the addition of tyrosine kinase inhibitor, imatinib.[4] [5] While imatinib has been linked to pneumatosis intestinalis in a child with acute leukemia, most clinical trials for childhood Ph+?ALL have not reported this as a significant toxicity.[4] [5] [6] Of interest was L-asparaginase, linked to pancreatitis in 6.7%?18% of children being treated for ALL.[7] The clinical course of drug-induced pancreatitis can vary from mild to severe and in our patient serum lipase returned to normal within 72?hours and CT abdomen showed no evidence of pseudocyst or necrosis, excluding severe pancreatitis as a cause of gastrointestinal perforation.[8] Gastrointestinal tract perforation is reportedly seen in less than 1% of patients on induction therapy for ALL.[9] L-asparaginase-related jejunal perforation has been described in a patient with ALL, with the etiology related to the prothrombotic state induced by reducing levels of natural anticoagulants such as protein C, protein S, Antithrombin III, and plasminogen.[10] [11] Also, imatinib has very rarely been reported to cause bowel perforation, but given the rarity and length of exposure it is unlikely to be the causative factor in our patient.[12] [13] [14]
Thrombotic complications are seen in 2%?7% of patients with ALL receiving asparaginase. The driving mechanism for thrombosis is related to the depletion of L-asparaginase-dependent hemostatic protein synthesis. Thrombotic events most often occur during induction and corticosteroids may contribute by increasing synthesis of procoagulants as well as by inducing vascular changes.[15] Majority of patients develop venous thrombosis, but arterial thrombosis has also been reported.[16] Thrombosis secondary to L-asparaginase is usually managed with LMWH. L-asparaginase may need temporary discontinuation in the presence of clinically significant thrombotic events, however, re-exposure is considered to be safe and feasible and is usually done under the cover of anticoagulation therapy.[3] Intracardiac thrombus amongst patients receiving L-asparaginase usually involves the right atrium in 2%?14% of children with ALL and is usually related to the presence of catheter tip in right atrium while the LV has not been described as a site for a thrombus.[17] [18] LV thrombosis has been described in patients with hypereosinophilic syndrome, as well as in a child with acquired protein C deficiency.[19] [20] Amongst adults, LV thrombus commonly occurs following myocardial infarction but has also occasionally been described amongst patients with cancer.[21] [22] [23] LV thrombus poses a risk of embolism resulting in ischemic stroke and peripheral embolism, because of which immediate anticoagulation therapy is recommended. In adults the preferred anticoagulation is usually oral warfarin along with low dose aspirin for 3 to 6 months.[23] Surgery is recommended if the general condition of the patient is preserved. Since our patient was a child who had undergone a major gastric surgery, he was commenced on subcutaneous LMWH and since he was severely neutropenic it was decided to defer any cardiac surgery until the time of count recovery.
Our patient did not have any past history of thrombotic episodes or family history of thrombophilia, but the co-occurrence of these unusual complications made us strongly suspect a underlying prothrombotic condition exacerbated by L-asparaginase therapy.[10] The prevalence of genetic prothrombotic abnormalities amongst children with ALL varies around the world, and we do not pre-emptively screen for thrombophilia given that such testing is expensive, and not easily available. The Dutch Children's Oncology Group has debated the benefit of more aggressive screening and LMWH prophylaxis during induction for those found to have thrombophilia.[24] The role of genetic predisposition for pancreatitis is less clear but recent genome-wide association studies have found different candidate single-nucleotide polymorphisms associated with pancreatitis in patients with ALL.[25] We could not rule out a pre-existing cardiac thrombus as a baseline 2D-echo was unavailable. Also, thrombotic events and gastric perforation during ALL therapy are often considered to be multifactorial rather than secondary to a single drug. But, the occurrence of several unique toxicities which are often shown to be associated with L-asparaginase, all occurring simultaneously in a patient would be the highlight of this case report.
Conclusion
Though L-asparaginase is an essential drug for the management of childhood ALL, it does possess a unique toxicity profile. Unfortunately, our patient simultaneously experienced several toxicities, including pancreatitis, LV thrombus, and gastrointestinal perforation leading to his demise. Lack of familiarity of the toxicity profile of this drug can make L-asparaginase a difficult drug to use. Being vigilant for these unusual toxicities especially during induction chemotherapy is essential for optimal patient care.
Conflict of interest
None declared.
Source of Support
None.
Declaration of Patient Consent
The authors certify that they have obtained all appropriate patient consent forms.
References
- ilverman LB, Gelber RD, Dalton VK. et al.?Improved outcome for children with acute lymphoblastic leukemia: results of Dana-Farber Consortium Protocol 91-01. Blood 2001; 97 (05) 1211-1218
- ?Gupta S, Wang C, Raetz EA. et al.?Impact of asparaginase discontinuation on outcome in childhood acute lymphoblastic leukemia: a report from the Children's Oncology Group. J Clin Oncol 2020; 38 (17) 1897-1905
- ?Hijiya N, van der Sluis IM.?Asparaginase-associated toxicity in children with acute lymphoblastic leukemia. Leuk Lymphoma 2016; 57 (04) 748-757
- ?Schultz KR, Bowman WP, Aledo A. et al.?Improved early event-free survival with imatinib in Philadelphia chromosome-positive acute lymphoblastic leukemia: a children's oncology group study. J Clin Oncol 2009; 27 (31) 5175-5181
- ?Biondi A, Schrappe M, De Lorenzo P. et al.?Imatinib after induction for treatment of children and adolescents with Philadelphia-chromosome-positive acute lymphoblastic leukaemia (EsPhALL): a randomised, open-label, intergroup study. Lancet Oncol 2012; 13 (09) 936-945
- ?O'Rafferty C, McElligott F, Storey L, O'Marcaigh A, Smith O.?Pneumatosis intestinalis and imatinib mesylate. Ann Hematol 2014; 93 (10) 1783-1784
- ?Raja RA, Schmiegelow K, Frandsen TL.?Asparaginase-associated pancreatitis in children. Br J Haematol 2012; 159 (01) 18-27
- ?Wolthers BO, Frandsen TL, Baruchel A. et al; Ponte di Legno Toxicity Working Group.?Asparaginase-associated pancreatitis in childhood acute lymphoblastic leukaemia: an observational Ponte di Legno Toxicity Working Group study. Lancet Oncol 2017; 18 (09) 1238-1248
- ?M?ricke A, Zimmermann M, Valsecchi MG. et al.?Dexamethasone vs prednisone in induction treatment of pediatric ALL: results of the randomized trial AIEOP-BFM ALL 2000. Blood 2016; 127 (17) 2101-2112
- ?Appel IM, Hop WC, van Kessel-Bakvis C, Stigter R, Pieters R.?L-Asparaginase and the effect of age on coagulation and fibrinolysis in childhood acute lymphoblastic leukemia. Thromb Haemost 2008; 100 (02) 330-337
- ?Tang ER, Chapman T, Finn LS, Leger KJ.?Perforated jejunitis in a child with acute lymphoblastic leukemia treated with pegaspargase. Radiol Case Rep 2018; 13 (03) 568-572
- ?El Jurdi N, Bankoff M, Klein A, Saif MW.?Perforation of the colon during imatinib mesylate (Gleevec) treatment in a patient with chronic myeloid leukemia (CML). Cureus 2016; 8 (06) e660
- ?Tadaiya MV, Tankshali RA.?Multiple small bowel perforations in a patient of chronic myeloid leukemia on imatinib. Indian J Med Paediatr Oncol 2020; 41: 89-92
- ?Chiarugi M, Galatioto C, Lippolis PV, Seccia M.?Multiple bowel perforations complicating imatinib treatment for advanced gastrointestinal stromal tumor. J Am Coll Surg 2008; 206 (02) 386-387
- ?Riley DO, Schlefman JM, Vitzthum Von Eckstaedt V HC, Morris AL, Keng MK, El Chaer F.?Pegaspargase in practice: minimizing toxicity, maximizing benefit. Curr Hematol Malig Rep 2021; 16 (03) 314-324
- ?Payne JH, Vora AJ.?Thrombosis and acute lymphoblastic leukaemia. Br J Haematol 2007; 138 (04) 430-445
- ?Athale UH, Chan AK.?Thrombosis in children with acute lymphoblastic leukemia: part I. Epidemiology of thrombosis in children with acute lymphoblastic leukemia. Thromb Res 2003; 111 (03) 125-131
- ?Mitchell LG, Andrew M, Hanna K. et al; Prophylactic Antithrombin Replacement in Kids with Acute Lymphoblastic Leukemia Treated with Asparaginase Group (PARKAA).?A prospective cohort study determining the prevalence of thrombotic events in children with acute lymphoblastic leukemia and a central venous line who are treated with L-asparaginase: results of the Prophylactic Antithrombin Replacement in Kids with Acute Lymphoblastic Leukemia Treated with Asparaginase (PARKAA) Study. Cancer 2003; 97 (02) 508-516
- ?Atalay S, Akar N, Tutar HE, Yilmaz E.?Factor V 1691 G-A mutation in children with intracardiac thrombosis: a prospective study. Acta Paediatr 2002; 91 (02) 168-171
- ?Williams E, Smart SC, Go RS.?Catastrophic thromboembolism in a patient with acute lymphoblastic leukemia and hypereosinophilia. Haematologica 2004; 89 (04) EIM01
- ?McCarthy CP, Vaduganathan M, McCarthy KJ, Januzzi Jr JL, Bhatt DL, McEvoy JW.?Left ventricular thrombus after acute myocardial infarction: screening, prevention, and treatment. JAMA Cardiol 2018; 3 (07) 642-649
- ?Oeser C, Andreas M, Rath C, Habertheuer A, Kocher A.?Left ventricular thrombus in a patient with cutaneous T-cell lymphoma, hypereosinophilia and?Mycoplasma pneumoniae?infection?a challenging diagnosis: a case report. J Cardiothorac Surg 2015; 10: 21
- ?Ikeda A, Yamachika E, Mizutani M. et al.?Rapid occurrence of left ventricular thrombus associated with platinum-based chemotherapy plus cetuximab for the treatment of metastatic squamous cell carcinoma of the head and neck: a case report. Mol Clin Oncol 2017; 7 (05) 833-836
- ?Klaassen ILM, Lauw MN, Fiocco M. et al.?Venous thromboembolism in a large cohort of children with acute lymphoblastic leukemia: risk factors and effect on prognosis. Res Pract Thromb Haemost 2019; 3 (02) 234-241
- Wolthers BO, Frandsen TL, Abrahamsson J. et al.?Asparaginase-associated pancreatitis: a study on phenotype and genotype in the NOPHO ALL2008 protocol. Leukemia 2017; 31 (02) 325-332
Address for correspondence
Publication History
21 April 2022 (online)
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

|?Fig. 1(A)CT abdomen showing left ventricular thrombus (arrow head) and pneumoperitoneum (*) (B) with gastric perforation (arrow). (C) 2D-echocardiography confirming the presence of left ventricular thrombus (outlined circle). (D) Intraoperative findings showing perforation of greater curvature of stomach (arrow head) (E) impending perforation of jejunum (double arrow).
References
- Gelber RD, Dalton VK. et al.?Improved outcome for children with acute lymphoblastic leukemia: results of Dana-Farber Consortium Protocol 91-01. Blood 2001; 97 (05) 1211-1218
- upta S, Wang C, Raetz EA. et al.?Impact of asparaginase discontinuation on outcome in childhood acute lymphoblastic leukemia: a report from the Children's Oncology Group. J Clin Oncol 2020; 38 (17) 1897-1905
- ijiya N, van der Sluis IM.?Asparaginase-associated toxicity in children with acute lymphoblastic leukemia. Leuk Lymphoma 2016; 57 (04) 748-757
- chultz KR, Bowman WP, Aledo A. et al.?Improved early event-free survival with imatinib in Philadelphia chromosome-positive acute lymphoblastic leukemia: a children's oncology group study. J Clin Oncol 2009; 27 (31) 5175-5181
- iondi A, Schrappe M, De Lorenzo P. et al.?Imatinib after induction for treatment of children and adolescents with Philadelphia-chromosome-positive acute lymphoblastic leukaemia (EsPhALL): a randomised, open-label, intergroup study. Lancet Oncol 2012; 13 (09) 936-945
- 'Rafferty C, McElligott F, Storey L, O'Marcaigh A, Smith O.?Pneumatosis intestinalis and imatinib mesylate. Ann Hematol 2014; 93 (10) 1783-1784
- aja RA, Schmiegelow K, Frandsen TL.?Asparaginase-associated pancreatitis in children. Br J Haematol 2012; 159 (01) 18-27
- olthers BO, Frandsen TL, Baruchel A. et al; Ponte di Legno Toxicity Working Group.?Asparaginase-associated pancreatitis in childhood acute lymphoblastic leukaemia: an observational Ponte di Legno Toxicity Working Group study. Lancet Oncol 2017; 18 (09) 1238-1248
- ?ricke A, Zimmermann M, Valsecchi MG. et al.?Dexamethasone vs prednisone in induction treatment of pediatric ALL: results of the randomized trial AIEOP-BFM ALL 2000. Blood 2016; 127 (17) 2101-2112
- Appel IM, Hop WC, van Kessel-Bakvis C, Stigter R, Pieters R.?L-Asparaginase and the effect of age on coagulation and fibrinolysis in childhood acute lymphoblastic leukemia. Thromb Haemost 2008; 100 (02) 330-337
- Tang ER, Chapman T, Finn LS, Leger KJ.?Perforated jejunitis in a child with acute lymphoblastic leukemia treated with pegaspargase. Radiol Case Rep 2018; 13 (03) 568-572
- El Jurdi N, Bankoff M, Klein A, Saif MW.?Perforation of the colon during imatinib mesylate (Gleevec) treatment in a patient with chronic myeloid leukemia (CML). Cureus 2016; 8 (06) e660
- Tadaiya MV, Tankshali RA.?Multiple small bowel perforations in a patient of chronic myeloid leukemia on imatinib. Indian J Med Paediatr Oncol 2020; 41: 89-92
- Chiarugi M, Galatioto C, Lippolis PV, Seccia M.?Multiple bowel perforations complicating imatinib treatment for advanced gastrointestinal stromal tumor. J Am Coll Surg 2008; 206 (02) 386-387
- Riley DO, Schlefman JM, Vitzthum Von Eckstaedt V HC, Morris AL, Keng MK, El Chaer F.?Pegaspargase in practice: minimizing toxicity, maximizing benefit. Curr Hematol Malig Rep 2021; 16 (03) 314-324
- Payne JH, Vora AJ.?Thrombosis and acute lymphoblastic leukaemia. Br J Haematol 2007; 138 (04) 430-445
- Athale UH, Chan AK.?Thrombosis in children with acute lymphoblastic leukemia: part I. Epidemiology of thrombosis in children with acute lymphoblastic leukemia. Thromb Res 2003; 111 (03) 125-131
- Mitchell LG, Andrew M, Hanna K. et al; Prophylactic Antithrombin Replacement in Kids with Acute Lymphoblastic Leukemia Treated with Asparaginase Group (PARKAA).?A prospective cohort study determining the prevalence of thrombotic events in children with acute lymphoblastic leukemia and a central venous line who are treated with L-asparaginase: results of the Prophylactic Antithrombin Replacement in Kids with Acute Lymphoblastic Leukemia Treated with Asparaginase (PARKAA) Study. Cancer 2003; 97 (02) 508-516
- Atalay S, Akar N, Tutar HE, Yilmaz E.?Factor V 1691 G-A mutation in children with intracardiac thrombosis: a prospective study. Acta Paediatr 2002; 91 (02) 168-171
- Williams E, Smart SC, Go RS.?Catastrophic thromboembolism in a patient with acute lymphoblastic leukemia and hypereosinophilia. Haematologica 2004; 89 (04) EIM01
- McCarthy CP, Vaduganathan M, McCarthy KJ, Januzzi Jr JL, Bhatt DL, McEvoy JW.?Left ventricular thrombus after acute myocardial infarction: screening, prevention, and treatment. JAMA Cardiol 2018; 3 (07) 642-649
- Oeser C, Andreas M, Rath C, Habertheuer A, Kocher A.?Left ventricular thrombus in a patient with cutaneous T-cell lymphoma, hypereosinophilia and?Mycoplasma pneumoniae?infection?a challenging diagnosis: a case report. J Cardiothorac Surg 2015; 10: 21
- Ikeda A, Yamachika E, Mizutani M. et al.?Rapid occurrence of left ventricular thrombus associated with platinum-based chemotherapy plus cetuximab for the treatment of metastatic squamous cell carcinoma of the head and neck: a case report. Mol Clin Oncol 2017; 7 (05) 833-836
- Klaassen ILM, Lauw MN, Fiocco M. et al.?Venous thromboembolism in a large cohort of children with acute lymphoblastic leukemia: risk factors and effect on prognosis. Res Pract Thromb Haemost 2019; 3 (02) 234-241
- Wolthers BO, Frandsen TL, Abrahamsson J. et al.?Asparaginase-associated pancreatitis: a study on phenotype and genotype in the NOPHO ALL2008 protocol. Leukemia 2017; 31 (02) 325-332


 PDF
PDF  Views
Views  Share
Share

