Normal Tissue Complications following Hypofractionated Chest Wall Radiotherapy in Breast Cancer Patients and Their Correlation with Patient, Tumor, and Treatment Characteristics
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(02): 121-127
DOI: DOI: 10.4103/ijmpo.ijmpo_80_16
Abstract
Introduction: Normal tissue complications following chest wall radiotherapy (RT) are inevitable, and the long-term data on hypofractionation are still limited. To quantify the late effects of hypofractionated RT on cardiac, pulmonary, brachial plexus, and regional lymphatics and their correlation with patient, tumor, and treatment characteristics is the main objective of this study. Materials and Methods: Two hundred and sixteen breast cancer patients following mastectomy were treated with hypofractionated schedules either 40 Gy in 15 fractions or 42.5 Gy in 16 fractions. Common Toxicity Criteria version 3.0 was utilized to quantify the late effects of hypofractionation on cardiac, pulmonary, brachial plexus, and lymphedema at a maximum follow-up of 5 years. Results: Median follow-up was 42 months. Median age was 49 years. 14.8%-developed ≥Grade (Gr) 2 late cardiac toxicity. 10.2%-developed ≥Gr2 late pulmonary toxicity. There were 28.7%-patients who developed ≥Gr2 lymphedema. Sixty-seven out of 216 patients had symptomatic brachial plexopathy at 5-year follow-up. Variables found to increase the incidence of these adverse events included smoking, hypertension, diabetes mellitus, body mass index ≥25, extent of axillary dissection, and use of supraclavicular field. Conclusion: Hypofractionation leads to increased risk of normal tissue complications partly influenced by some patient- and treatment-related factors, but these were manageable and minimally disabling.
Publication History
Article published online:
06 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Introduction:
Normal tissue complications following chest wall radiotherapy (RT) are inevitable, and the long-term data on hypofractionation are still limited. To quantify the late effects of hypofractionated RT on cardiac, pulmonary, brachial plexus, and regional lymphatics and their correlation with patient, tumor, and treatment characteristics is the main objective of this study.
Materials and Methods:
Two hundred and sixteen breast cancer patients following mastectomy were treated with hypofractionated schedules either 40 Gy in 15 fractions or 42.5 Gy in 16 fractions. Common Toxicity Criteria version 3.0 was utilized to quantify the late effects of hypofractionation on cardiac, pulmonary, brachial plexus, and lymphedema at a maximum follow-up of 5 years.
Results:
Median follow-up was 42 months. Median age was 49 years. 14.8%-developed ≥Grade (Gr) 2 late cardiac toxicity. 10.2%-developed ≥Gr2 late pulmonary toxicity. There were 28.7%-patients who developed ≥Gr2 lymphedema. Sixty-seven out of 216 patients had symptomatic brachial plexopathy at 5-year follow-up. Variables found to increase the incidence of these adverse events included smoking, hypertension, diabetes mellitus, body mass index ≥25, extent of axillary dissection, and use of supraclavicular field.
Conclusion:
Hypofractionation leads to increased risk of normal tissue complications partly influenced by some patient- and treatment-related factors, but these were manageable and minimally disabling.
Introduction
Radiation plays an important role in the management of breast cancer. Postmastectomy radiotherapy (RT) is highly effective in preventing chest wall recurrences.[1] Postoperative RT to chest wall after mastectomy is usually given in 25 fractions of 2 Gy fraction size in 5 weeks followed by a boost if needed. Hypofractionated RT, whereby a fraction size of >2 Gy is utilized, has gained significant popularity as adjuvant treatment postbreast conserving surgery, based on the results of various randomized trials.[2,3,4,5] However, the data on the use of postmastectomy hypofractionated RT and its effects on normal tissues are still limited.
Based on the radiobiological predictions, it is clear that the use of >2 Gy produces unfavorable long-term sequelae. Adhering to the radiobiological principles, it has been quantified that the healthy tissues of breast and underlying structures are sensitive to fraction size, volume irradiated, and total dose delivered. Using linear quadratic model, α/β values for late reacting tissues are found to be 5 Gy or less.[6] α/β ratio is the dose D at which the components of cell killing that are proportional to dose, i.e., αD equal those that are proportional to the square of dose, i.e., βD2. Therefore, even small changes in fraction sizes can produce relatively large changes in effects of RT on the late-reacting tissues. Postmastectomy, chest wall irradiation exposes organs such as lungs and heart to a significant amount of radiation which increases the normal tissue complications. These complications are inevitable, and given the long-term survival of breast cancer patients, these long-term adverse effects should be quantified. Moreover, although new modalities such as intensity-modulated RT and three-dimensional RT have come a long way in sparing normal tissues, use of two-dimensional treatment with cobalt 60 is still prevalent in developing countries like ours.
Using postmastectomy hypofractionated RT in conjunction with conventional planning can impair normal tissue function in the long run. To quantify the late effects of hypofractionated RT on pulmonary, cardiac, brachial plexus, and lymphedema sequelae is the main objective of this study, and to find out the correlation of patient, tumor, and treatment characteristics with these late effects is the secondary objective to carry out this study.
Materials and Methods
Patients
A total of 216 female patients with breast cancer following mastectomy were followed from January 2011 to January 2016. Study was approved by the Local Ethics Committee. Eligibility criteria include female patients, postmastectomy, with histologically proven disease, >20 years age group with either stage 1–3. All patients with breast-conserving surgery, positive margins, chest malformations, chronic cardiac or pulmonary disease, pregnant or lactating were excluded from evaluation.
Radiotherapy
Hypofractionated schedules used were either 42.5 Gy in 16 fractions with 2.65 Gy fraction in 22 days or 40 Gy in 15 fractions with 2.66 Gy fraction in 19 days depending on physician's preference. RT was delivered by Theratron 780 teletherapy cobalt 60 machine, with the intention to treat chest wall along with regional lymphatics depending on the histopathology report. Tangential RT portals were utilized for chest wall RT and direct portal for supraclavicular/axillary fields. Bolus was used on alternate days. Two-dimensional planning was done.
Patient assessment
Echocardiography and pulmonary function tests (PFTs) were done for all patients before the start of RT and then after 3 monthly intervals till 1 year, followed by 6 monthly assessments till last follow-up. Percentage decline in ejection fraction was used to evaluate cardiac reactions, while for pulmonary function, measurements were recorded as percentages of predicted values. For lymphedema, grading percentage change in inter-limb discrepancy in volume or circumference at point of greatest visible difference in conjunction with other points such as obscuration of anatomic architecture, pitting edema, and interference with daily life was taken into account. And finally, for brachial plexopathy, grading patient's symptomatology along with active daily life score was used. This grading is in accordance with the Common Terminology Criteria for Adverse Events version 3.0.
Statistical analysis
Descriptive statistics were used to present the data. For repeated measurements, analysis of variance was utilized to assess the difference in function values at different times and to correlate the respective function values at different times with multiple demographic variables. Pearson Chi-square test was used to assess correlation of numerical variables.
Results
Patient characteristics
A total of 216 patients were followed from January 2011 to January 2016 for the effects of hypofractionated RT on normal tissue toxicity especially pertaining to cardiac, pulmonary, brachial plexus, and lymphedema sequelae in postmastectomy breast cancer patients. Median follow-up was 42 months. The majority of the patients were in 40–60 years age group, with median age of 49 years [Table 1]. Sixteen percent of the females were either active or ex-smokers. Twenty-two percent had diabetes, and 24.5% were known hypertensive. The majority had stage two or three disease with ductal histology being more common. Axillary dissection was done in 175 patients with adequate dissection meaning more than equal to ten nodes dissected was performed in 117 patients. All patients were given full course of chemotherapy before the start of RT. Tamoxifen was prescribed as hormonal therapy to 103 patients, while 39 were given aromatase inhibitor (either anastrozole or letrozole). The supraclavicular field was prescribed to 131 patients based on their histopathological features.
Table 1
Patient, tumor, and treatment characteristics

|
Toxicity analysis
We mainly restricted our analysis to late adverse events which are described as those occurring >6 months after the completion of RT [Table 2]. Our main organs of interest were heart, lungs, brachial plexus, and lymphedema, because these are the main organs at risk during chest wall irradiation, and their long-term toxicity adversely affects the quality of life of an individual.
Table 2
Toxicity analysis (n=216)
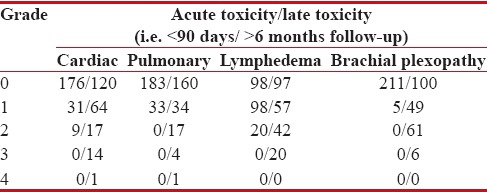
|
Cardiac
Thirty-two out of 216 patients developed ≥ Grade (Gr) 2 late cardiac toxicity, and out of which, one patient had congestive cardiac failure which was poorly controlled by medication.
Pulmonary
About 10.2% of patients had ≥Gr2 late pulmonary toxicity. Although symptomatic pneumonitis was rare in our study, 5 patients had clinically significant percentage decline in PFTs over their predicted values.
Lymphedema
Arm circumference was taken 5 cm above and below the olecranon and at wrist level and was compared with the opposite arm. Sixty-two patients developed >10% inter-limb discrepancy accompanied with easily observed swelling or obscuration of anatomic structures. Twenty patients had lymphedema which interfered with their active daily life.
Brachial plexopathy
Clinically symptomatic/significant brachial plexopathy was observed in 12.5% of the patients. Only six patients developed brachial plexopathy interfering with active daily life. There were none with disabling brachial plexopathy observed after a median follow-up of 42 months.
Discussion
Hypofractionated RT was introduced to ease the burden of patients and hospitals alike. However, use of hypofractionation leads to unacceptably high percentage of severe complications.[7,8,9] Fractional size of >2 Gy produces unacceptable late adverse sequelae.[10] Due to their close proximity to chest wall, certain organs/structures suffer radiation-induced toxicities. These late adverse effects can significantly impact the quality of life in long run. Opposed tangential portals with or without supraclavicular field expose structures such as heart, lungs, brachial plexus, and regional lymphatics to significant amounts of radiation doses. Moreover, all these structures are particularly sensitive to fractionation. As for this study, keeping in view the late reactions, the biologically effective doses delivered with two schedules came out to be 80.3 Gy for 40 Gy in 15 fractions and 75.3 Gy for 42.5 Gy in 16 fractions.
To add to this, there are certain patient-, disease-, and treatment-related factors which influence the risk of chest wall irradiation such as age, personal history of patient, extent of nodal dissection, number of nodes dissected, use of supraclavicular/axillary field, and so on. These factors have been shown by a number of studies to increase the incidence of normal tissue complications. However, the majorities were carried out in conjunction with conventional fractionation regimes. There is very scarce data on the relevance of these factors in the era of hypofractionated RT, especially in the postmastectomy settings, where the likelihood of normal tissue complications increases with time. Moreover, to collect data on how these factors influence the effects of hypofractionated RT on normal tissue functions was also one of the aims of this study.
First, for cardiac tissues [Table 3], we reported an incidence of 14.8% of ≥Gr2 late cardiac toxicity, with statistically significant difference when left-sided disease was compared with the right side (P < 0 xss=removed>et al.[11] study, who reported an increased risk of cardiac deaths after treatment for left-sided breast cancer. Age at irradiation has been demonstrated to increase the risk. Hooning et al.[12] reported that younger age <35>45 years. King et al.[13] showed that factors such as hypertension, being overweight, and diabetes influence the overall risk of radiation-induced heart injury. We too observed that 21 out of 47 patients with diabetes and 25 out of 53 hypertensives suffered ≥Gr2 cardiac toxicity with results being statistically significant. Furthermore, patients with body mass index (BMI) ≥25 had statistically significant late adverse effects. Smoking also increases the risk, with 60% of active/ex-smokers developing ≥Gr2 cardiac toxicity. Radiation potentiates the cardiotoxic effects of some chemotherapeutic agents such as anthracyclines, and this interaction is dose dependent.[14] The total cumulative dose of anthracycline in our study was well below 360 mg/m2. Our trial found no statistically significant late adverse effect in patients receiving chemotherapy, with no influence of the type of regime utilized. Concerning hormonal therapy, no statistically significant difference was found.
Table 3
Cardiac toxicity in association with demographic variables
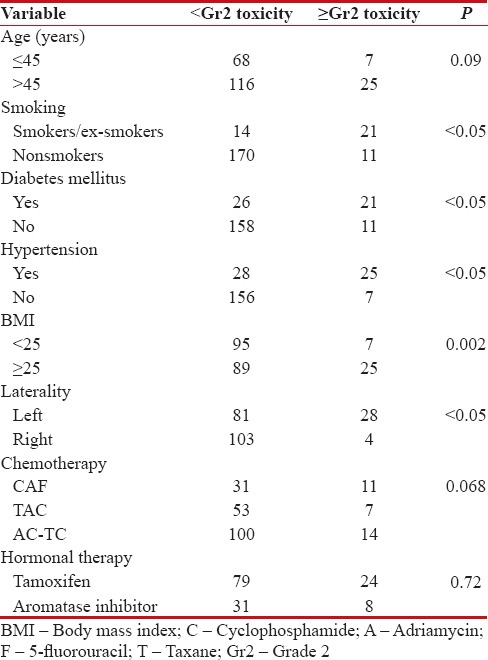
|
Second, for lung tissues [Table 4], we observed 10.2% of late ≥Gr2 adverse events at 42 months of median follow-up. Lind et al.[15] reported that use of axillary/supraclavicular portals in addition to tangential portals increased the incidence of pulmonary complications. We reported statistically significant incidence of late pulmonary toxicity in those who received supraclavicular RT (P = 0.03) as compared to those who did not. Age and smoking have also been considered as risk factors contributing to radiation-induced lung injury.[16,17,18,19,20,21] In our series, although we observed no statistically significant effect of age on pulmonary adverse effects, smoking definitely potentiates the side effect of hypofractionation on pulmonary functions with 17 out of 35 smokers/ex-smokers suffering ≥Gr2 toxicity, the results being statistically significant (P < 0 xss=removed>et al.[22] found increased incidence of radiation-induced lung injury in patients receiving chemotherapy. The impact of hormonal therapy on radiation pneumonitis has also been demonstrated in a number of trials.[23,24] In our study, we found that both chemotherapy and hormonal therapy do increase the risk of lung injury when combined with hypofractionated RT. Being diabetic or hypertensive also increased the risk of pulmonary toxicity with statistically significant results for ≥Gr2 late lung toxicity.
Table 4
Pulmonary toxicity in association with demographic variables
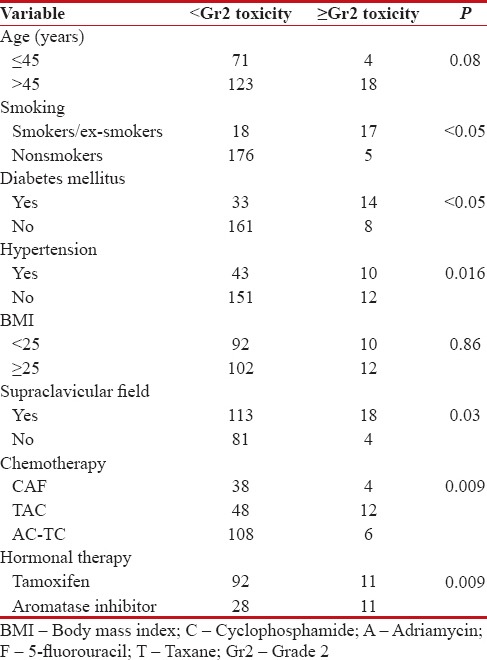
|
Concerning lymphedema, various studies quote 5%–42% rate of lymphedema following breast cancer treatment.[25] Risk increases when axillary dissection is combined with axillary RT. In our study, we reported the risk of clinically significant ≥Gr2 lymphedema at 28.7% [Table 5], and this risk was more in patients who underwent axillary dissection (57 out of 175), and especially increased in those who had ≥10 lymph nodes dissected (P = 0.0005). Other risk factors associated with increased incidence of lymphedema included smoking (P < 0 xss=removed>P < 0 xss=removed>P < 0 xss=removed>et al.[26] found that being obese increased the risk of arm edema in patients. Böhler et al.[27] in another series described hypertension as the risk factor for lymphedema after axillary surgery and RT. Adjuvant chemotherapy and hormonal therapy were not found to influence the risk of lymphedema in our study.
Table 5
Association of lymphedema with different demographic variables
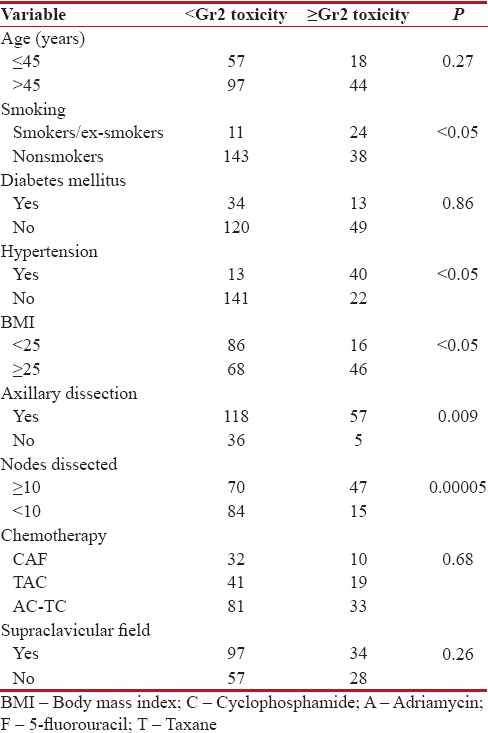
|
Finally, regarding brachial plexopathy, there is a concern that hypofractionated schedules may be associated with the higher risk of late complications to the brachial plexus. The risk of developing brachial plexopathy after conventionally fractionated megavoltage RT is estimated to be below 1%.[28,29] Galecki et al.[30] in a review article estimated that the risk of brachial plexopathy ranged from 1.7% up to 73% with the use of doses per fraction in the range from 2.2 to 4.58 Gy with the total doses between 43.5 and 60 Gy. They also concluded that surgical manipulations in the axilla and chemotherapy have to be taken into account as additional factors which may increase the risk of brachial plexopathy. In our present study, we observed an overall incidence of 30.6% [Table 6] for any Gr of brachial plexopathy at a maximum follow-up of 5 years, with 12.5% incidence of ≥Gr2 brachial plexopathy. The extent of axillary dissection in conjunction with use of supraclavicular field significantly influenced the appearance of clinically symptomatic brachial plexopathy with P < 0 xss=removed>P = 0.00002), diabetes mellitus (P < 0 xss=removed>P = 0.007).
Table 6
Association of brachial plexopathy with different variables
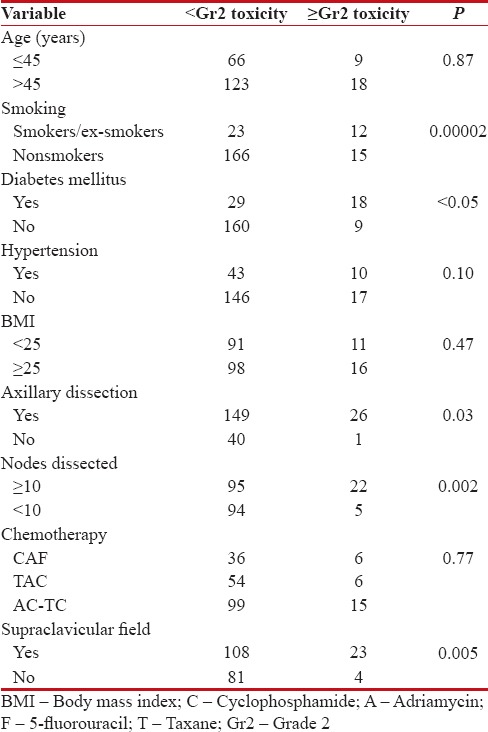
|
Hypofractionated schedules have gained a great impetus in present times following the publications of Standardisation of Breast Radiotherapy A and B trials. These studies have equated hypofractionation with conventional regime following breast conservation therapy with limited data in postmastectomy settings. Although the normal tissues studied in this trial are sensitive to fractionation with α/β values of ≤3 Gy, we observed clinically few to none disabling adverse events even at a maximum follow-up period of 5 years. However, there are certain risk factors pertaining to patient, disease, and treatment such as smoking, hypertension, diabetes mellitus, BMI, extent of axillary dissection, and use of supraclavicular/axillary field and hormonal therapy, which significantly influence the development of cardiac, pulmonary, lymphedema, and brachial plexopathy and late adverse effects. Moreover, although these adverse events were symptomatic and interfered with active daily life of patient, they are manageable.
Conclusion
Results from this study clearly support the use of hypofractionated RT regimes for chest wall irradiation in postmastectomy settings with no fear of disabling/nonmanageable long-term normal tissue complications although more longer follow-up data are still warranted.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Fletcher GH. Clinical dose response curve of subclinical aggregates of epithelial cells and its practical application in the management of human cancer. In: Friedman M, editor. Biological and Clinical Basis of Radiosensitivity. Springfield, IL: Charles C. Thomas; 1974. p. 485.
- START Trialists' Group, Bentzen SM, Agrawal RK, Aird EG, Barrett JM, Barrett-Lee PJ, et al. The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: A randomised trial. Lancet Oncol 2008;9:331-41.
- START Trialists' Group, Bentzen SM, Agrawal RK, Aird EG, Barrett JM, Barrett-Lee PJ, et al. The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: A randomised trial. Lancet 2008;371:1098-107.
- Owen JR, Ashton A, Bliss JM, Homewood J, Harper C, Hanson J, et al. Effect of radiotherapy fraction size on tumour control in patients with early-stage breast cancer after local tumour excision: Long-term results of a randomised trial. Lancet Oncol 2006;7:467-71.
- Whelan T, MacKenzie R, Julian J, Levine M, Shelley W, Grimard L, et al. Randomized trial of breast irradiation schedules after lumpectomy for women with lymph node-negative breast cancer. J Natl Cancer Inst 2002;94:1143-50.
- Thames HD, Bentzen SM, Turesson I, Overgaard M, Van den Bogaert W. Time-dose factors in radiotherapy: A review of the human data. Radiother Oncol 1990;19:219-35.
- Cox JD. Large-dose fractionation (hypofractionation). Cancer 1985;55 9 Suppl: 2105-11.
- Langberg CW, Hauer-Jensen M. Influence of fraction size on the development of late radiation enteropathy. An experimental study in the rat. Acta Oncol 1996;35:89-94.
- Wang EH, Sekyi-Otu A, O'Sullivan B, Bell RS. Management of long-term postirradiation periclavicular complications. J Surg Oncol 1992;51:259-65.
- Bates T, Evans RG. Report of the Independent Review Commissioned by the Royal College of Radiologists into Brachial Plexus Neuropathy Following Radiotherapy for Breast Carcinoma. London: Royal College of Radiologists; 1995.
- Darby S, McGale P, Peto R, Granath F, Hall P, Ekbom A. Mortality from cardiovascular disease more than 10 years after radiotherapy for breast cancer: Nationwide cohort study of 90 000 Swedish women. BMJ 2003;326:256-7.
- Hooning MJ, Botma A, Aleman BM, Baaijens MH, Bartelink H, Klijn JG, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst 2007;99:365-75.
- King V, Constine LS, Clark D, Schwartz RG, Muhs AG, Henzler M, et al. Symptomatic coronary artery disease after mantle irradiation for Hodgkin's disease. Int J Radiat Oncol Biol Phys 1996;36:881-9.
- Shapiro CL, Hardenbergh PH, Gelman R, Blanks D, Hauptman P, Recht A, et al. Cardiac effects of adjuvant doxorubicin and radiation therapy in breast cancer patients. J Clin Oncol 1998;16:3493-501.
- Lind PA, Rosfors S, Wennberg B, Glas U, Bevegård S, Fornander T. Pulmonary function following adjuvant chemotherapy and radiotherapy for breast cancer and the issue of three-dimensional treatment planning. Radiother Oncol 1998;49:245-54.
- Ooi GC, Kwong DL, Ho JC, Lock DT, Chan FL, Lam WK, et al. Pulmonary sequelae of treatment for breast cancer: A prospective study. Int J Radiat Oncol Biol Phys 2001;50:411-9.
- Lind PA, Marks LB, Hardenbergh PH, Clough R, Fan M, Hollis D, et al. Technical factors associated with radiation pneumonitis after local +/regional radiation therapy for breast cancer. Int J Radiat Oncol Biol Phys 2002;52:137-43.
- Johansson S, Bjermer L, Franzen L, Henriksson R. Effects of ongoing smoking on the development of radiation-induced pneumonitis in breast cancer and oesophagus cancer patients. Radiother Oncol 1998;49:41-7.
- Yu TK, Whitman GJ, Thames HD, Buzdar AU, Strom EA, Perkins GH, et al. Clinically relevant pneumonitis after sequential paclitaxel-based chemotherapy and radiotherapy in breast cancer patients. J Natl Cancer Inst 2004;96:1676-81.
- Bentzen SM, Skoczylas JZ, Overgaard M, Overgaard J. Radiotherapy-related lung fibrosis enhanced by tamoxifen. J Natl Cancer Inst 1996;88:918-22.
- Wennberg B, Gagliardi G, Sundbom L, Svane G, Lind P. Early response of lung in breast cancer irradiation: Radiologic density changes measured by CT and symptomatic radiation pneumonitis. Int J Radiat Oncol Biol Phys 2002;52:1196-206.
- Lingos TI, Recht A, Vicini F, Abner A, Silver B, Harris JR. Radiation pneumonitis in breast cancer patients treated with conservative surgery and radiation therapy. Int J Radiat Oncol Biol Phys 1991;21:355-60.
- Azria D, Belkacemi Y, Romieu G, Gourgou S, Gutowski M, Zaman K, et al. Concurrent or sequential adjuvant letrozole and radiotherapy after conservative surgery for early-stage breast cancer (CO-HO-RT): A phase 2 randomised trial. Lancet Oncol 2010;11:258-65.
- Recht A. Radiotherapy, antihormonal therapy, and personalised medicine. Lancet Oncol 2010;11:215-6.
- Norman SA, Localio AR, Potashnik SL, Simoes Torpey HA, Kallan MJ, Weber AL, et al. Lymphedema in breast cancer survivors: Incidence, degree, time course, treatment, and symptoms. J Clin Oncol 2009;27:390-7.
- Segerström K, Bjerle P, Graffman S, Nyström A. Factors that influence the incidence of brachial oedema after treatment of breast cancer. Scand J Plast Reconstr Surg Hand Surg 1992;26:223-7.
- Böhler FK, Rhomberg W, Doringer W. Hypertension as risk factor for increased rate of side effects in the framework of breast carcinoma irradiation. Strahlenther Onkol 1992;168:344-9.
- Fowble BL, Solin LJ, Schultz DJ, Goodman RL. Ten year results of conservative surgery and irradiation for stage I and II breast cancer. Int J Radiat Oncol Biol Phys 1991;21:269-77.
- Pierce SM, Recht A, Lingos TI, Abner A, Vicini F, Silver B, et al. Long-term radiation complications following conservative surgery (CS) and radiation therapy (RT) in patients with early stage breast cancer. Int J Radiat Oncol Biol Phys 1992;23:915-23.
- Galecki J, Hicer-Grzenkowicz J, Grudzien-Kowalska M, Michalska T, Zalucki W. Radiation-induced brachial plexopathy and hypofractionated regimens in adjuvant irradiation of patients with breast cancer – A review. Acta Oncol 2006;45:280-4.
References
- Fletcher GH. Clinical dose response curve of subclinical aggregates of epithelial cells and its practical application in the management of human cancer. In: Friedman M, editor. Biological and Clinical Basis of Radiosensitivity. Springfield, IL: Charles C. Thomas; 1974. p. 485.
- START Trialists' Group, Bentzen SM, Agrawal RK, Aird EG, Barrett JM, Barrett-Lee PJ, et al. The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: A randomised trial. Lancet Oncol 2008;9:331-41.
- START Trialists' Group, Bentzen SM, Agrawal RK, Aird EG, Barrett JM, Barrett-Lee PJ, et al. The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: A randomised trial. Lancet 2008;371:1098-107.
- Owen JR, Ashton A, Bliss JM, Homewood J, Harper C, Hanson J, et al. Effect of radiotherapy fraction size on tumour control in patients with early-stage breast cancer after local tumour excision: Long-term results of a randomised trial. Lancet Oncol 2006;7:467-71.
- Whelan T, MacKenzie R, Julian J, Levine M, Shelley W, Grimard L, et al. Randomized trial of breast irradiation schedules after lumpectomy for women with lymph node-negative breast cancer. J Natl Cancer Inst 2002;94:1143-50.
- Thames HD, Bentzen SM, Turesson I, Overgaard M, Van den Bogaert W. Time-dose factors in radiotherapy: A review of the human data. Radiother Oncol 1990;19:219-35.
- Cox JD. Large-dose fractionation (hypofractionation). Cancer 1985;55 9 Suppl: 2105-11.
- Langberg CW, Hauer-Jensen M. Influence of fraction size on the development of late radiation enteropathy. An experimental study in the rat. Acta Oncol 1996;35:89-94.
- Wang EH, Sekyi-Otu A, O'Sullivan B, Bell RS. Management of long-term postirradiation periclavicular complications. J Surg Oncol 1992;51:259-65.
- Bates T, Evans RG. Report of the Independent Review Commissioned by the Royal College of Radiologists into Brachial Plexus Neuropathy Following Radiotherapy for Breast Carcinoma. London: Royal College of Radiologists; 1995.
- Darby S, McGale P, Peto R, Granath F, Hall P, Ekbom A. Mortality from cardiovascular disease more than 10 years after radiotherapy for breast cancer: Nationwide cohort study of 90 000 Swedish women. BMJ 2003;326:256-7.
- Hooning MJ, Botma A, Aleman BM, Baaijens MH, Bartelink H, Klijn JG, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst 2007;99:365-75.
- King V, Constine LS, Clark D, Schwartz RG, Muhs AG, Henzler M, et al. Symptomatic coronary artery disease after mantle irradiation for Hodgkin's disease. Int J Radiat Oncol Biol Phys 1996;36:881-9.
- Shapiro CL, Hardenbergh PH, Gelman R, Blanks D, Hauptman P, Recht A, et al. Cardiac effects of adjuvant doxorubicin and radiation therapy in breast cancer patients. J Clin Oncol 1998;16:3493-501.
- Lind PA, Rosfors S, Wennberg B, Glas U, Bevegård S, Fornander T. Pulmonary function following adjuvant chemotherapy and radiotherapy for breast cancer and the issue of three-dimensional treatment planning. Radiother Oncol 1998;49:245-54.
- Ooi GC, Kwong DL, Ho JC, Lock DT, Chan FL, Lam WK, et al. Pulmonary sequelae of treatment for breast cancer: A prospective study. Int J Radiat Oncol Biol Phys 2001;50:411-9.
- Lind PA, Marks LB, Hardenbergh PH, Clough R, Fan M, Hollis D, et al. Technical factors associated with radiation pneumonitis after local +/regional radiation therapy for breast cancer. Int J Radiat Oncol Biol Phys 2002;52:137-43.
- Johansson S, Bjermer L, Franzen L, Henriksson R. Effects of ongoing smoking on the development of radiation-induced pneumonitis in breast cancer and oesophagus cancer patients. Radiother Oncol 1998;49:41-7.
- Yu TK, Whitman GJ, Thames HD, Buzdar AU, Strom EA, Perkins GH, et al. Clinically relevant pneumonitis after sequential paclitaxel-based chemotherapy and radiotherapy in breast cancer patients. J Natl Cancer Inst 2004;96:1676-81.
- Bentzen SM, Skoczylas JZ, Overgaard M, Overgaard J. Radiotherapy-related lung fibrosis enhanced by tamoxifen. J Natl Cancer Inst 1996;88:918-22.
- Wennberg B, Gagliardi G, Sundbom L, Svane G, Lind P. Early response of lung in breast cancer irradiation: Radiologic density changes measured by CT and symptomatic radiation pneumonitis. Int J Radiat Oncol Biol Phys 2002;52:1196-206.
- Lingos TI, Recht A, Vicini F, Abner A, Silver B, Harris JR. Radiation pneumonitis in breast cancer patients treated with conservative surgery and radiation therapy. Int J Radiat Oncol Biol Phys 1991;21:355-60.
- Azria D, Belkacemi Y, Romieu G, Gourgou S, Gutowski M, Zaman K, et al. Concurrent or sequential adjuvant letrozole and radiotherapy after conservative surgery for early-stage breast cancer (CO-HO-RT): A phase 2 randomised trial. Lancet Oncol 2010;11:258-65.
- Recht A. Radiotherapy, antihormonal therapy, and personalised medicine. Lancet Oncol 2010;11:215-6.
- Norman SA, Localio AR, Potashnik SL, Simoes Torpey HA, Kallan MJ, Weber AL, et al. Lymphedema in breast cancer survivors: Incidence, degree, time course, treatment, and symptoms. J Clin Oncol 2009;27:390-7.
- Segerström K, Bjerle P, Graffman S, Nyström A. Factors that influence the incidence of brachial oedema after treatment of breast cancer. Scand J Plast Reconstr Surg Hand Surg 1992;26:223-7.
- Böhler FK, Rhomberg W, Doringer W. Hypertension as risk factor for increased rate of side effects in the framework of breast carcinoma irradiation. Strahlenther Onkol 1992;168:344-9.
- Fowble BL, Solin LJ, Schultz DJ, Goodman RL. Ten year results of conservative surgery and irradiation for stage I and II breast cancer. Int J Radiat Oncol Biol Phys 1991;21:269-77.
- Pierce SM, Recht A, Lingos TI, Abner A, Vicini F, Silver B, et al. Long-term radiation complications following conservative surgery (CS) and radiation therapy (RT) in patients with early stage breast cancer. Int J Radiat Oncol Biol Phys 1992;23:915-23.
- Galecki J, Hicer-Grzenkowicz J, Grudzien-Kowalska M, Michalska T, Zalucki W. Radiation-induced brachial plexopathy and hypofractionated regimens in adjuvant irradiation of patients with breast cancer – A review. Acta Oncol 2006;45:280-4.


 PDF
PDF  Views
Views  Share
Share

