Papillary Tumor of the Pineal Region: A Case with Unique Immunohistochemical Keratin Expression Pattern
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2020; 41(03): 412-414
DOI: DOI: 10.4103/ijmpo.ijmpo_191_18
Abstract
Papillary tumor of the pineal region (PTPR) is a rare neuroepithelial tumor first described in 2003. It is a newly recognized entity and introduced in the WHO classification of central nervous system tumors in 2007. Till date, about 70 cases have been described in literature. Herein, we report an additional case of PTPR in a 25-year-old male with emphasis on immunohistochemistry and ultrastructural features. Interestingly, this case showed a unique membranous as well as dot-like Golgi zone keratin (AE1/AE3 and CK18) expression pattern.
Publication History
Received: 31 August 2018
Accepted: 29 September 2019
Article published online:
28 June 2021
© 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Papillary tumor of the pineal region (PTPR) is a rare neuroepithelial tumor first described in 2003. It is a newly recognized entity and introduced in the WHO classification of central nervous system tumors in 2007. Till date, about 70 cases have been described in literature. Herein, we report an additional case of PTPR in a 25-year-old male with emphasis on immunohistochemistry and ultrastructural features. Interestingly, this case showed a unique membranous as well as dot-like Golgi zone keratin (AE1/AE3 and CK18) expression pattern.
Introduction
Papillary tumor of the pineal region (PTPR) is a rare neuroepithelial tumor arising from specialized ependyma of the subcommissural organ (SCO). PTPR was first described by Jouvet et al. in 2003.[1] It is a newly recognized entity and introduced in the WHO classification of central nervous system tumors in 2007.[2] It commonly occurs in adults, though age ranges from 5 to 66 years with a slight female predominance.[3] Headache is the most common presentation due to increased cerebrospinal fluid pressure resulting from compression of the cerebral aqueduct. Herein, we report a case of PTPR in a 25-year-old male with emphasis on immunohistochemistry and ultrastructural features.
Case Report
A 25-year-old male presented to the neurosurgery outpatient department with the chief complaints of gradual-onset and slowly progressive headache associated with intermittent episodes of vomiting for 5 months. The patient also noted diminution of vision in both the eyes for 1 month. A single episode of loss of consciousness for 15 min was elucidated. No history of neurological deficits or seizures was present. Magnetic resonance imaging (MRI) showed a heterogeneously enhancing mass below the splenium of corpus callosum invading into the posterior 3rd ventricle compressing tectal plate inferiorly and compromising cerebral aqueduct, measuring 50 mm × 28 mm × 24 mm and suggestive of pineoblastoma [Figure 1]. Anterior 3rd and both the lateral ventricles were dilated. A midline suboccipital craniotomy, infratentorial supracerebellar approach, and tumor decompression were done. Postoperatively, the patient could not be weaned off ventilation; later, his condition deteriorated, and he succumbed to cardiac arrest on the 4th day.
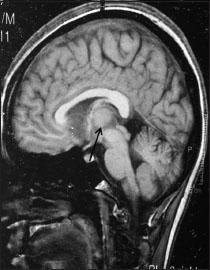
| Figure 1:Magnetic resonance imaging T1-weighted contrast image of sagittal section showing a heterogeneously enhancing mass below the splenium of corpus callosum invaginating into the posterior 3rd ventricle compressing tectal plate inferiorly and compromising cerebral aqueduct likely a pineal mass measuring 50 mm × 28 mm × 24 mm in maximum anteroposterior, cephalocaudal, and lateral dimensions, respectively
Histopathology, immunohistochemistry, and ultrastructural features
The tumor consisted of cells arranged as diffuse solid sheets and forming occasional pseudorosettes and many papillary structures. The tumor cells were small with a moderate amount of cytoplasm, oval to round nuclei with dispersed chromatin, and mostly inconspicuous nucleoli [Figure 2]a. Occasional mitosis was noted [Figure 2]b. No necrosis, atypia, or microvascular proliferation was seen. The tumor cells were positive for both epithelial and mesenchymal markers including strong and diffuse positivity for pancytokeratin (AE1/AE3), CK18, vimentin, and S-100 and focally for synaptophysin, glial fibrillary acidic protein (GFAP), and epithelial membrane antigen [Figure 2]c, [Figure 2]d, [Figure 2]e, [Figure 2]f, [Figure 2]g, [Figure 2]h,[Figure 2]i. The tumor cells were negative for chromogranin-A, neurofilament protein, CK7, CK20, and CK5/6. A MIB-1 labeling index of 2%–3%-was noted. On electron microscopy, these cells delineated microvilli and cilia similar to ependymoma, which confirmed their origin from the SCO of the pineal region [Figure 3]a, [Figure 3]b.
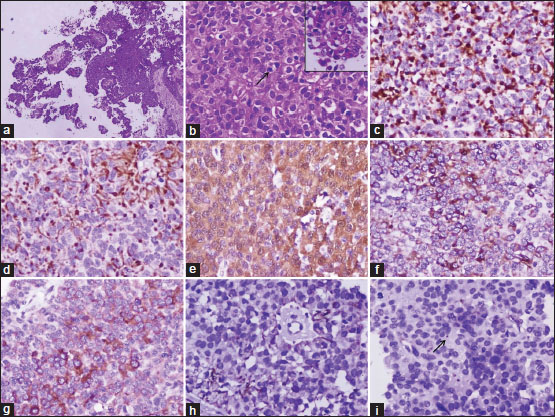
| Figure.2:Photomicrographs showing (a) tumor cells arranged in a diffuse sheet and papillary fronds (H and E × 400), (b) arrow showing mitotic figure with inset highlighting papillary fronds (H and E × 400), (c) AE1/AE3 and (d) CK18 stains showing both membranous and dot positivity (×400), (e) S-100 stain showing both nuclear and cytoplasmic positivity (×400), (f) vimentin stain showing cytoplasmic positivity (×400), (g) synaptophysin stain showing cytoplasmic positivity (×400), (h) glial fibrillary acidic protein stain showing focal fibrillar positivity (×400), (i) epithelial membrane antigen stain showing occasional dot-like positivity (arrow) (×400)
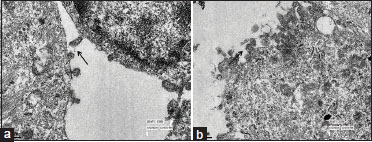
| Figure.3:Electron microscopic pictures (a and b) showing numerous cilia and microvilli in the surface lining (arrow) (×50,000)
Discussion
PTPR is a rare tumor of the pineal region. Since its first description, about 70 cases have been reported mainly as case reports and short case series. PTPR presents as a circumscribed mass with a well-delineated border with adjacent pineal gland and brain parenchyma. On computer tomography, it appears as a hypodense contrast-enhancing mass, whereas MRI shows hyperintensity on T2-weighted sequence and enhancement on T1-weighted sequences with gadolinium. However, these imaging findings are not a characteristic diagnostic feature for PTPR.
The PTPRs are thought to arise from the remnants of specialized ependymal cells of the subcommissural organ, which is supported by the tumor location, immunohistochemical expression of nestin and CK18, and ultrastructural features.[3]
As the name indicates, light microscopy shows characteristic papillary fronds admixed with variable amount of solid components comprising pseudorosettes, true rosettes, and tubules with lumina. The fibrovascular core of the papillary fronds may be hyalinized and covered with pseudostratified columnar epithelial lining. Mitotic activity is variable. Necrosis may be seen. In our case, occasional mitoses 1–2/10 hpf was noted; however, necrosis was absent.
PTPRs show a characteristic pattern of expression for keratins, which is more prominent in papillaroid fragments. A wide keratin panel including AE1/AE3, CAM5.2, KL1, and CK18 are usually positive. Some cases may also show focal positivity for CK7 and CK5/6; however, CK20 remains negative. Our case showed both membranous and unique dot-like Golgi zone positivity for AE1/AE3 and CK18, whereas it was negative for CK7 and CK5/6. Although dot positivity for EMA is described, this case exceptionally showed keratin expression in the form of dot-like positivity in the Golgi zone.
Other nonepithelial markers such as S-100, vimentin, synaptophysin, neuron-specific enolase, chromogranin, and GFAP with variable expression are also described.[4] This case showed focal positivity for synaptophysin, S-100, vimentin, and GFAP. Epithelial membrane antigen (EMA) is an infrequently expressed marker, which may show dot-like positivity similar to ependymoma. We found occasional cells displaying dot-like EMA expression [Figure 2]i.
Many tumors of the pineal region can present with papillary architecture. The two major differential diagnoses are papillary ependymoma and choroid plexus tumors. Choroid plexus tumors usually show CK7 positivity and CK 20 negativity. Choroid plexus tumors can be confirmed by Kir-7.1 and stanniocalcin-1 staining.
Papillary ependymomas show many overlapping features on light microscopy and ultrastructurally, but a more diffuse GFAP and EMA positivity and rare expression of CK distinguish it from PTPR.
At present, not enough data are available to form clinical guidelines for the treatment of PTPR. Rickard et al.[5] in their analysis of 59 PTPR patients found description of treatment modalities in 56 patients, in which surgery was used alone in 23.2%, surgery with radiotherapy in 42.9%, and surgery plus radiotherapy and chemotherapy in 30.3%-of patients. The prognosis of PTPR is uncertain. Frequent local recurrence and occasional spinal dissemination is described. In 2007 WHO classification, PTPR is graded as either Grade II or III and definite grading could not be done.
Fèvre-Montange et al.[6] in their series of 31 patients found progression in 72%-of the cases with a 5-year overall survival and progression-free survival in 73%- and 27%-of the cases, respectively. Only gross total resection was found to be associated with overall survival and recurrence. Heim et al.[7] found that increased mitotic and proliferative activities are associated with worse prognosis in PTPR.
To conclude, we describe an additional case of PTPR with an interesting keratin expression patterns (both membranous and dot-like Golgi zone positivity) and a highlight on ultrastructural features. Unfortunately, this patient succumbs postoperatively during hospital course without receiving any chemo- or radiotherapy.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Conflict of Interest
There are no conflicts of interest.
References
- Jouvet A, Fauchon F, Liberski P, Saint-Pierre G, Didier-Bazes M, Heitzmann A. et al. Papillary tumor of the pineal region. Am J Surg Pathol 2003; 27: 505-12
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK. WHO Classification of Tumours of the Central Nervous System Revised. Lyon: IARC 2016; 4: 180-2
- Roncaroli F, Scheithauer BW. Papillary tumor of the pineal region and spindle cell oncocytoma of the pituitary: New tumor entities in the 2007 WHO classification. Brain Pathol 2007; 17: 314-8
- Hasselblatt M, Blümcke I, Jeibmann A, Rickert CH, Jouvet A, van de NesJA. et al. Immunohistochemical profile and chromosomal imbalances in papillary tumours of the pineal region. Neuropathol Appl Neurobiol 2006; 32: 287-83
- Rickard KA, Parker JR, Vitaz TW, Plaga AR, Wagner S, Parker JCJr. et al. Papillary tumor of the pineal region: Two case studies and a review of the literature. Ann Clin Lab Sci 2011; 41: 174-81
- Fèvre-Montange M, Hasselblatt M, Figarella-Branger D, Chauveinc L, Champier J, Saint-Pierre G. et al. Prognosis and histopathologic features in papillary tumors of the pineal region: A retrospective multicenter study of 31 cases. J Neuropathol Exp Neurol 2006; 65: 1004-11
- Heim S, Beschorner R, Mittelbronn M, Keyvani K, Riemenschneider MJ, Vajtai I. et al. Increased mitotic and proliferative activity are associated with worse prognosis in papillary tumors of the pineal region. Am J Surg Pathol 2014; 38: 106-10
Address for correspondence
Publication History
Received: 31 August 2018
Accepted: 29 September 2019
Article published
online:
28 June 2021
© 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301
UP, India

| Figure 1:Magnetic resonance imaging T1-weighted contrast image of sagittal section showing a heterogeneously enhancing mass below the splenium of corpus callosum invaginating into the posterior 3rd ventricle compressing tectal plate inferiorly and compromising cerebral aqueduct likely a pineal mass measuring 50 mm × 28 mm × 24 mm in maximum anteroposterior, cephalocaudal, and lateral dimensions, respectively

| Figure.2:Photomicrographs showing (a) tumor cells arranged in a diffuse sheet and papillary fronds (H and E × 400), (b) arrow showing mitotic figure with inset highlighting papillary fronds (H and E × 400), (c) AE1/AE3 and (d) CK18 stains showing both membranous and dot positivity (×400), (e) S-100 stain showing both nuclear and cytoplasmic positivity (×400), (f) vimentin stain showing cytoplasmic positivity (×400), (g) synaptophysin stain showing cytoplasmic positivity (×400), (h) glial fibrillary acidic protein stain showing focal fibrillar positivity (×400), (i) epithelial membrane antigen stain showing occasional dot-like positivity (arrow) (×400)

| Figure.3:Electron microscopic pictures (a and b) showing numerous cilia and microvilli in the surface lining (arrow) (×50,000)
References
- Jouvet A, Fauchon F, Liberski P, Saint-Pierre G, Didier-Bazes M, Heitzmann A. et al. Papillary tumor of the pineal region. Am J Surg Pathol 2003; 27: 505-12
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK. WHO Classification of Tumours of the Central Nervous System Revised. Lyon: IARC 2016; 4: 180-2
- Roncaroli F, Scheithauer BW. Papillary tumor of the pineal region and spindle cell oncocytoma of the pituitary: New tumor entities in the 2007 WHO classification. Brain Pathol 2007; 17: 314-8
- Hasselblatt M, Blümcke I, Jeibmann A, Rickert CH, Jouvet A, van de NesJA. et al. Immunohistochemical profile and chromosomal imbalances in papillary tumours of the pineal region. Neuropathol Appl Neurobiol 2006; 32: 287-83
- Rickard KA, Parker JR, Vitaz TW, Plaga AR, Wagner S, Parker JCJr. et al. Papillary tumor of the pineal region: Two case studies and a review of the literature. Ann Clin Lab Sci 2011; 41: 174-81
- Fèvre-Montange M, Hasselblatt M, Figarella-Branger D, Chauveinc L, Champier J, Saint-Pierre G. et al. Prognosis and histopathologic features in papillary tumors of the pineal region: A retrospective multicenter study of 31 cases. J Neuropathol Exp Neurol 2006; 65: 1004-11
- Heim S, Beschorner R, Mittelbronn M, Keyvani K, Riemenschneider MJ, Vajtai I. et al. Increased mitotic and proliferative activity are associated with worse prognosis in papillary tumors of the pineal region. Am J Surg Pathol 2014; 38: 106-10


 PDF
PDF  Views
Views  Share
Share

