Pediatric Differentiated Thyroid Carcinoma Risk Factor For Analysis For Disease Free Survival
CC BY-NC-ND 4.0 ? Indian J Med Paediatr Oncol 2018; 39(02): 153-158
DOI: DOI: 10.4103/ijmpo.ijmpo_70_17
Abstract
Context:The context of this study was epidemiology pediatric thyroid cancer in Bandung, Indonesia. Aims: The aim of this study is to evaluate clinical characteristics and outcome between children and young adult patients with differentiated thyroid cancer (DTC) treated in our hospital.?Settings and Design:This was a cohort retrospective study.?Materials and Methods:?The medical records of 144 patients with DTC who underwent thyroid surgery followed by radioiodine and thyroid hormone suppression were retrospectively reviewed. Thyroid cancers were diagnosed between January 2007 and December 2010. Participants consisted of 43 patients who were younger than 21 years old and 101 young adult patients (older than 21 years old but younger or equal to 40 years). The clinical characteristics and outcomes were analyzed and compared, and then, recurrence-free survival was evaluated using Kaplan?Meier methods. Statistical Analysis Used: Software R 3.3.0 version for Windows was used in this study.?Results:?Female has higher tendency to have thyroid cancer than male (P = 0.006). Based on histopathology report, classic papillary thyroid cancer is the most common cancer type in children than young adult. However, there was no significant difference between two groups regarding thyroid cancer size and multifocality (P = 0.815 and P = 0.370). The risk of recurrent ratio of children to young adults is 3.88 (95% confidence interval [CI] 1.38; 10.91). A similar result trend has been shown for sex type, histopathology type, number of nodules, surgical technique, and metastasis parameters (adjusted hazard ratio = 7.91, 95% CI 2.11; 29.67).?Conclusions:?DTC in children shows more aggressive behavior compared to young adult patients.
Publication History
Article published online:
23 June 2021
? 2018. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Context:The context of this study was epidemiology pediatric thyroid cancer in Bandung, Indonesia. Aims: The aim of this study is to evaluate clinical characteristics and outcome between children and young adult patients with differentiated thyroid cancer (DTC) treated in our hospital.?Settings and Design:This was a cohort retrospective study.?Materials and Methods:?The medical records of 144 patients with DTC who underwent thyroid surgery followed by radioiodine and thyroid hormone suppression were retrospectively reviewed. Thyroid cancers were diagnosed between January 2007 and December 2010. Participants consisted of 43 patients who were younger than 21 years old and 101 young adult patients (older than 21 years old but younger or equal to 40 years). The clinical characteristics and outcomes were analyzed and compared, and then, recurrence-free survival was evaluated using Kaplan?Meier methods. Statistical Analysis Used: Software R 3.3.0 version for Windows was used in this study.?Results:?Female has higher tendency to have thyroid cancer than male (P = 0.006). Based on histopathology report, classic papillary thyroid cancer is the most common cancer type in children than young adult. However, there was no significant difference between two groups regarding thyroid cancer size and multifocality (P = 0.815 and P = 0.370). The risk of recurrent ratio of children to young adults is 3.88 (95% confidence interval [CI] 1.38; 10.91). A similar result trend has been shown for sex type, histopathology type, number of nodules, surgical technique, and metastasis parameters (adjusted hazard ratio = 7.91, 95% CI 2.11; 29.67).?Conclusions:?DTC in children shows more aggressive behavior compared to young adult patients.
Introduction
Although thyroid cancer is less common in pediatrics than adult patients, the incidence of pediatric thyroid cancer has increased gradually in Indonesia as well as throughout the world. Thyroid cancer has become the fifth most common cancer in children age of 0?14 years and the most common cancer in adolescents and young adults. Cancer screening tests for early detection of thyroid cancer have been implicated as the impact of its rise in adult thyroid cancer. However, because children and adolescents generally do not undergo such test, genetic or environmental factors have been suggested as possible causes of the increased incidence of pediatric thyroid cancer.[1],[2]
The clinicopathological characteristics and outcomes of thyroid cancers in adults were recently published. Pediatric thyroid cancers tend to be at more advanced stages at the time of diagnosis and have a higher frequency of recurrences than adulthood thyroid cancers. However, it remains unclear whether the pathology diagnosis and long-term outcomes differ between children and adolescent thyroid cancer patients.[3]
Furthermore, despite their advanced pathological presentations, pediatric patients have better prognosis and significantly lower mortality rates than adult ones. This finding reveals that, even in similarly advanced stage at the time of diagnosis, long-term outcome and prognosis may differ between pediatrics and adult patients. As the prognostic implications of the pathology according to age at diagnosis are unknown, no age-related optimal-clinical practice guidelines for the treatment and monitoring of thyroid cancer in pediatric patients are available. The comparison between pediatrics and adult patients with thyroid cancer in this study may help to develop age-related optimal management and follow-up guidelines in the future.[4]
We investigated changes in the clinic pathological presentation and long-term outcomes of pediatric thyroid cancer according to age at diagnosis during the year of 2009?2011 and compare the clinicopathological predictors of recurrence-free survival (RFS) between pediatrics and adult patients with differentiated thyroid cancer (DTC).
Materials and Methods
Materials
The medical records of 144 children and young adult patients (<40>
From those, 43 of participants were below 21 years of age and categorized as pediatric patients. The pediatric age should be limited to 21 years as from pragmatic point of view, our centers may transfer pediatric patients between 18 and 21 years of age to adult care and manage those children under pediatric guidelines until transition period has been completed.[5] 101 (one hundred one) young adult patients with thyroid cancer were recorded. Eight patients with intermediate histopathology such as tall cell and insular carcinoma were excluded from the analysis. Clinicopathological characteristics that include age, sex, primary tumor size, multimodality therapy, extrathyroidal extension (ETE), lymph node (LN), and/or distant metastasis at diagnosis were investigated. The long-term outcomes of 43 pediatric patients' data were assessed and compared to those of 101 of young adults. All patients had been followed up for 60 months after multimodality treatment. This analysis was using patients from the Oncology, Head and Neck Surgery Department, Hasan Sadikin General Hospital, and the patients' list was obtained from individual review of hospital archives, registry, and databases. Data base was differentiated patients' treatment diagnosed from January 1, 2007, to December 31, 2011.
In addition, specific categories were included to specify disease progression. A clinical measurement was taken before or at the time of surgery if the pathologic measurement was not available. Distant metastases were identified most commonly by plain chest X-ray or whole-body scan. All patients should at least have a 1-year follow-up from initial treatment.
Methods
Disease progression was chosen as the end point of analysis due to many patients already had metastatic disease at diagnosis. We consider disease progression if there was local recurrence recognized within thyroid bed or regional LNs after a complete removal, progression in the thyroid bed or regional nodes after incomplete surgical resection, and the development or progression of distant metastatic disease (lung and bone). Independent variables were age, ETE of primary tumor into surrounding tissue, primary tumor size, number of nodules, regional LN involvement, presence of distant metastases, the technique of initial surgery, the use of RAI, and histopathology type were assessed for their influence on disease progression.
Initial thyroid surgery was divided into three groups: (1) lobectomy and isthmectomy, (2) total thyroidectomy, and (3) total thyroidectomy + comprehensive neck dissection.
Morbidity during treatment was assessed by determining the association of significant wound complications, permanent recurrent nerve paralysis, permanent hypocalcemia with the extent of thyroid surgery, or nodal dissection. Wound complication included serous hemorrhage or hematoma (requiring exploration), infection, pneumothorax, and requirement for tracheostomy. Permanent hypoparathyroidism was presumed if there was a postoperative need for calcium supplements and/or Vitamin D replacement for >6 months after surgery and continued until the last follow-up. Permanent recurrent nerve paralysis was defined by change in voice and/or indirect laryngoscope evidence of vocal cord paralysis that lasted at least 6 months after the primary thyroid surgery. This included cases of operations that sacrificed the recurrent nerve. Temporary recurrent nerve injury or hypoparathyroidism resolved within 6 months of surgery. The pediatric patient data were then compared to those of 101 young adult patients with differentiated thyroid carcinoma.
Results
The average age at diagnosis of thyroid cancer was 18 years old (interquartile range [IQR] 8.8). The pathological finding papillary thyroid carcinoma (PTC) was 91% in pediatrics versus 66% in young adult. The average rate of size was 4.65 cm (IQR 1.10), and multifocality was found in 9 patients (21%). Metastasis in children was found in 5 patients (12%) while there were 10 patients (10%) in young adults. Total thyroidectomy was the most common procedure to treat DTC in both groups (n?= 37, 86% and n = 88, 87%). The report of operative injury to laryngeal nerve revealed in one pediatric patient (2%) and none in young adult patient. Hypocalcaemia was common after surgery and was the most common complication in pediatric group (n?= 9.18%), presumably due to more fragile anatomy structure in children's laryngeal nerves than ones in the adult [Table 1]. Some traction caused spasm to anterior thyroid artery and temporary hypocalcemia due to parathyroid gland failure. Addition of RAI treatment seemed not to have correlation with recurrence for both groups.
|
Characteristic |
Children (n=43) |
Young adult (n=101) |
Total (n=144) |
p |
|---|---|---|---|---|
|
*Independent t-test; ?Wilcoxon rank-sum test; $Chi-square test; #Fisher?s exact test. IQR ? Interquartile range; SD ? Standard deviation; RND ? Radical neck dissection; FTC ? Follicular thyroid cancer; PTC ? Papillary thyroid carcinoma; RAI ? Radioactive iodine; FTCV ? Follicular Thyroid Carcinoma Variant; PTCV: Papillary Thyroid Carcinoma Variant |
||||
|
Gender, frequency (%) |
||||
|
Female |
29 (67) |
88 (87) |
117 (81) |
0.006$ |
|
Male |
14 (33) |
13 (13) |
27 (19) |
|
|
Histopathology type, frequency (%) |
||||
|
FTC |
0 |
7 (7) |
7 (5) |
0.012# |
|
FTCV |
0 |
1 (1) |
1 (1) |
|
|
PTC |
39(91) |
67 (66) |
106 (74) |
|
|
PTCV |
4 (9) |
26 (26) |
30 (21) |
|
|
Nodule |
||||
|
Number, frequency (%) |
||||
|
Single |
34 (79) |
86 (85) |
120 (83) |
0.370$ |
|
Multiple |
9 (21) |
15 (15) |
24 (17) |
|
|
Size (cm) |
||||
|
For single, median (IQR) |
4.65 (1.10) |
4.30 (1.48) |
4.30 (1.23) |
0.815? |
|
For multiple, mean (SD) |
||||
|
First |
4.64 (1.22) |
5.33 (2.24) |
5.08 (1.92) |
0.407* |
|
Second |
4.13 (0.71) |
4.75 (1.49) |
4.52 (1.27) |
0.189* |
|
Metastasis, frequency (%) |
||||
|
Yes |
5 (12) |
10 (10) |
15 (10) |
0.770# |
|
No |
38 (88) |
91 (90) |
129 (90) |
|
|
Surgical procedure, frequency (%) |
||||
|
Isthmo-lobectomy |
3 (7) |
5 (5) |
8 (5) |
0.919# |
|
Total thyroidectomy |
37 (86) |
88 (87) |
125 (87) |
|
|
Total thyroidectomy with RND |
3 (7) |
8 (8) |
11 (8) |
|
|
Injury of Recurrent Laryngeal Nerve, frequency (%) |
||||
|
Yes |
1 (2) |
0 |
1 (1) |
0.299# |
|
No |
42 (98) |
101 (100) |
143 (99) |
|
|
Hypocalcemia after surgery, frequency (%) |
||||
|
Yes |
8 (19) |
1 (1) |
9 (6) |
<0> |
|
No |
35 (81) |
100 (99) |
135 (94) |
|
|
RAI treatment, frequency (%) |
||||
|
Yes |
39 (91) |
88 (87) |
127 (88) |
0.544$ |
|
No |
4 (9) |
13 (13) |
17 (12) |
|
|
Variable |
Crude HR (95% CI) |
P |
Adjusted HR (95% CI) |
|||
|---|---|---|---|---|---|---|
|
Model 1 |
P |
Model 2 |
P |
|||
|
HR ? Hazard ratio; CI ? Confidence interval; RAI ? Radioactive iodine |
||||||
|
Age group |
3.88 (1.38-10.91) |
0.010 |
7.91 (2.11-29.67) |
0.002 |
7.11 (2.10-24.04) |
0.002 |
|
Gender |
0.71 (0.20-2.51) |
0.594 |
1.14 (0.28-4.65) |
0.858 |
- |
- |
|
Histopathology type |
2.13 (0.48-9.42) |
0.321 |
0.70 (0.13-3.64) |
0.668 |
- |
- |
|
Number of nodule |
1.18 (0.33-4.17) |
0.802 |
0.98 (0.24-4.05) |
0.978 |
- |
- |
|
Isthmo-lobectomy |
22.62 (6.88-74.36) |
<0> |
98.25 (17.40-554.77) |
<0> |
87.56 (18.34-418.13) |
<0> |
|
Metastasis |
4.63 (1.58-13.55) |
0.005 |
8.69 (2.42-31.18) |
0.001 |
8.08 (2.45-26.71) |
0.001 |
|
Hypocalcemia |
7.81 (2.47-24.69) |
<0> |
- |
- |
- |
- |
|
RAI treatment |
0.18 (0.06-0.56) |
0.003 |
- |
- |
- |
- |
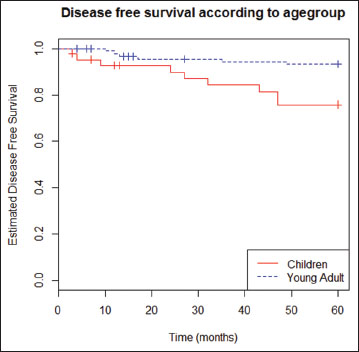
|?Figure.1Kaplan?Meir curves of disease-free recurrent in pediatric age groups and young adult age groups
Discussion
Previous guidelines for the management of thyroid cancers were geared toward adults. Compared to thyroid neoplasm in adults, those in the pediatric population exhibit differences in pathophysiology, clinical presentation, and long-term outcomes.[6],[7] Furthermore, therapy that may be recommended for an adult may not be appropriate for children who are at low risk for death but at higher risk for long-term harm caused by overly aggressive treatment. For these reasons, unique guidelines for children and adolescent with thyroid tumors are needed. Several studies have compared the clinical presentation and outcomes for children diagnosed with DTC <10 href="https://www.thieme-connect.com/products/ejournals/html/10.4103/ijmpo.ijmpo_70_17#JR_8" xss=removed>8]
In this study, there was a significant difference between children group and young adult one. Recurrence risk in thyroid cancer for pediatric patients was higher in young adult group compared to children with HR 3.88 at confidence interval of 95%. This study is consistent with the research of Lee?et al.[8]
In addition, there was a specific characteristic in pediatric thyroid cancer in children for the presence of multiple nodules. Lee?et al. study revealed a close relationship between multiple nodules with the recurrence risk. In our study, multiple nodules were dominantly found in children group patient, rather than in adult case, accounted for 21% and 15%, respectively. However, this was not statistically proven as significant factors to regulate the recurrence in the future.[8]
Other important role, apart from the age of patients, the presence of metastasis in LNs is important to be observed. If the neck LN is present, the possibility of locoregional recurrence will be more prevalent. This is confirmed with Nobuyuki?et al. study in 2009.[9]
Furthermore, treatment regimens vary which may impact outcomes. For example, surgeons may less aggressive in LN dissection in younger children, and this factor, rather than age, may impact recurrence rates. In our studies, we found that younger age was associated with an increased risk of recurrent nodal disease and lung metastases after adjustment for other risk factors.
There are uncertain factors to clinically predict the recurrence. Many of practitioners tend to avoid conducting radical operation on children's thyroid carcinomas. However, operation of only one side of thyroid (lobectomy with or without isthmus) that contains relatively small size tumor will be against by the group of surgeons who apply radical operation of thyroidectomy in thyroid cancer, even conducting prophylactic central LN dissection for tumor which size extend 1 cm as discussed by Chen?et al.[10]
This is understandable as in our research, LN metastasis is an accurate predictor factor to expect recurrence. Clinical research also agrees to importantly pursue the aggressiveness predictor factor on children's thyroid cancers. Studies by Conzo?et al. support this assumption.[11]
Nixon and Barczynski recommended routine central node dissection to prevent long-term recurrence and decrease postoperative thyroglobulin levels, citing the high risk of cervical LN metastasis.[12],[13] In contrast, Giordano?et al. summarized that this procedure increases the risk of postoperative complications such as hypothyroidism or recurrent laryngeal nerve injury, without any demonstrable long-term survival benefits.[14],[15]
Result from our research also shows that the neck LN metastasis presentation is the strong predictor factor to indicate recurrence. Therefore, we support the aggressive therapy. However, we feel that this should be supported by molecular biology study to seek predictor that can be used as reference for the aggressive therapy application.
Compared to adults, DTC in childhood is characterized by higher prevalence of gene rearrangements and lower frequency of point mutations in proto-oncogenes. Recent molecular studies have shown that BRAF mutation is the most common molecular abnormality in adults (36%-83% of cases) while this is rare in children.[16]
In contrast to the adults' PTC, PTC on children's molecular pathogenesis occurs sporadically in which 80% of them related to RET gene mutation, following the process of realignment with other genes, i.e., H4 and Elei that formed oncogenes RET/PTC. These genes encode proteins that play a role in the kinase tyrosine pathway in cells of the thyroid gland that is the path of mitogen-activated protein kinase (MAPK). to date, there are at least 11 different type of RET/PTC. All resulting from the fusion of tyrosine kinase domain of the 5' portion of different genes. RET/PTC1 and RET/PTC3 are the most common type, accounting for more than 90% of all re-arrangement. PTC that is caused by mutations in RET/PTC1 is more common in the age group above 20 years with subtype classic PTC, with tumors grow relatively slowly and occur sporadically, whereas mutations in RET/PTC3 are more common in the age group under 20 years old and have aggressive biological characteristic usually as tall cell variant PTC and a history of radiation exposure associated with the head and neck area as happened in Chernobyl and Nagasaki-Hiroshima.[17]
In general, the aggressiveness of a tumor is characterized by increasing proliferation and the ability of tumor cells to migrate out of the primary tumor to the other organs. This process is known as metastasis. At children and young adults PTC, allegedly increased proliferation caused by gene mutations and realignment of RET/PTC that will activate the MAPK pathway. RET/PTC, respectively, phosphorylate proteins that work in the MAPK pathway, ranging from Ras, Raf, MEK, and ERK. The active ERK proteins undergo translocation into the cell nucleus to activate the transcription factor that will stimulate the transcription process by promoters of genes that play a role in the proliferation.[18]
The ability of tumor cells to migrate begins with the unchain of bonds with neighboring cells and change in the cell skeleton or framework. Change in the framework of the cell causes the cell to penetrate the extracellular matrix and induce the transcription factors that alter epithelial cells into mesenchymal cells. This process is a key to the progression of all the cancer cells derived from epithelial. Integrity between epithelial structures with each other and between epithelial and basement membrane is barrier to prevent the occurrence of epithelial mesenchymal transition (EMT). The integrity of the cells is adhered by E-cadherin strong bound which forms the actin framework of the cell. Loss of E-cadherin bonding among these cells will disrupt desmosomes that maintain ties of inner filaments' order to prevent the cells, penetrating the extracellular matrix.. Epithelial cells are transformed into mesenchymal cells which have the ability to transcribe the factors that can degrade extracellular matrices such as matrix metalloproteinase. Formed mesenchymal cells also have the ability to stimulate synergy of protein signal that stimulates the formation of cancers' epithelial cell such as epidermal growth factor, hepatocyte growth factor, and fibroblast growth factor family, such as transforming growth factor-? (TGF-?).[18],[19]
On children and young adults' PTC, TGF-? RII role is suspected in reducing the expression of E-cadherin. Excessive expression of TGF-? RII can be activated by TGF-? produced by the thyroid tumor cells themselves or as product of other cells. The subsequent activity of SMAD pathway leads to activation of transcription factors, such as SNAIL that will stimulate E-cadherin gene promoter. The process that occurs is a corepressor to the transcription so that the expression of E-cadherin decreased.[20]
It can be concluded that fundamental protein in the beginning of process is E-cadherin, given proof that this protein expression changes will affect the expression of other proteins. In other words, E-cadherin acts as a conductor in an orchestra, and the orchestra is an EMT so that the expression of E-cadherin can represent EMT. If EMT occurs, then tumor cells will be able to move to other organs, showing those tumor cells are more aggressive. Hence, the aggressiveness of children and young adults' PTC can be represented by EMT.
This theory needs further research. However, if this is relevant, then biology molecular can be considered to determine therapy management on papillary thyroid cancer in children.
Conclusion
The risk of recurrent ratio of children to young adults is 3.88 (95% confidence interval [CI] 1:38; 10.91) meaning that children are more at risk to have recurrence compared to young adults. Similarly, after sex type, histopathology type, number of nodules, surgical technique, and metastasis (model 2) controlling, the conclusion remains the same (adjusted HR = 7.91, 95% CI 2.11, 29.67). DTC in children presents more aggressive behavior than in young adults patients.
Acknowledgment
The authors would like to thank Kurnia Wahyudi MD., MSc staff of Clinical Epidemiology and Biostatistics, Faculty of Medicine, Universitas Padjadjaran, Bandung, for providing statistical analysis.
Conflict of Interest
There are no conflicts of interest.
References
- oltz MM, Enomoto L, Ornstein R, Saunders B, Hollenbeak C.?Incidence and survival differences of differentiated thyroid cancer among younger women. Clin Oncol Adolesc Young Adults 2013; 3: 79-88
- a Vecchia C, Malvezzi M, Bosetti C, Garavello W, Bertuccio P.?et al.?Thyroid cancer mortality and incidence: A global overview. Int J Cancer 2015; 136: 2187-95
- haukar DA, Vaidya AD. Pediatric thyroid cancers: An Indian perspective?Indian J Surg Oncol?2012; 3:?166-72
- ung W, Sarlis NJ.?Current controversies in the management of pediatric patients with well-differentiated nonmedullary thyroid cancer: A review. Thyroid 2002; 12: 683-702
- rancis GL, Waguespack SG, Bauer AJ, Angelos P, Benvenga S.?et al.?Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid 2015; 25: 716-59
- ordioli MI, Moraes L, Cury AN, Cerutti JM.?Are we really at the dawn of understanding sporadic pediatric thyroid carcinoma?. Endocr Relat Cancer 2015; 22: R311-24
- hozeimeh N, Gingalewski C. Thyroid nodules in children: A single institution's experience.J. Oncol.2011;2011: 974125. Published online 2011 Oct 6.doi: 10.1155/2011/974125
- ee YA, Jung HW, Kim HY, Choi H, Kim HY, Hah JH.?et al.?Pediatric patients with multifocal papillary thyroid cancer have higher recurrence rates than adult patients: A retrospective analysis of a large pediatric thyroid cancer cohort over 33 years. J Clin Endocrinol Metab 2015; 100: 1619-29
- ada N, Sugino K, Mimura T, Nagahama M, Kitagawa W, Shibuya H. et al.?Pediatric differentiated thyroid carcinoma in stage I: Risk factor analysis for disease free survival. 2009; 9: 306
- Chen Q, Zou XH, Wei T, Huang QS, Sun YH.?Prediction of ipsilateral and contralateral central lymph node metastasis in unilateral papillary thyroid carcinoma: A retrospective study. Gland Surg 2015; 4: 288-94
- Conzo G, Docimo G, Pasquali D, Mauriello C, Gambardella C, Esposito D.?et al.?Predictive value of nodal metastases on local recurrence in the management of differentiated thyroid cancer. Retrospective clinical study. BMC Surg 2013; 13 Suppl2: S3
- Nixon IJ, Ganly I, Patel SG, Morris LG, Palmer FL, Thomas D.?Observation of clinically negative central compartment lymph nodes in papillary thyroid carcinoma. Surgery 2013; 154: 1166-72
- Barczy?ski M, Konturek A, Stopa M, Nowak W.?Prophylactic central neck dissection for papillary thyroid cancer. Br J Surg 2013; 100: 410-8
- Giordano D, Valcavi R, Thompson GB, Pedroni C, Renna L, Gradoni P.?et al.?Complications of central neck dissection in patients with papillary thyroid carcinoma: Results of a study on 1087 patients and review of the literature. Thyroid 2012; 22: 911-7
- Chisholm EJ, Kulinskaya E, Tolley NS. Systematic review and meta-analysis of the adverse effects of thyroidectomy combined with central neck dissection as compared with thyroidectomy alone?Laryngoscope?2009; 119:?1135-9
- Givens DJ, Buchmann LO, Agarwal AM, Grimmer JF, Hunt JP. BRAF V600E does not predict aggressive features of pediatric papillary thyroid carcinoma?Laryngoscope?2014; 124:?E389-93
- Fenton CL, Lukes Y, Nicholson D, Dinauer CA, Francis GL, Tuttle RM.?The ret/PTC mutations are common in sporadic papillary thyroid carcinoma of children and young adults. J Clin Endocrinol Metab 2000; 85: 1170-5
- Chapnick DA, Warner L, Bernet J, Rao T, Liu X. Partners in crime: The TGF? and MAPK pathways in cancer progression?Cell Biosci?2011; 1:?42
- Buehler D, Hardin H, Shan W, Montemayor-Garcia C, Rush PS, Asioli S. et al.?Expression of epithelial-mesenchymal transition regulators SNAI2 and TWIST1 in thyroid carcinomas. Mod Pathol 2013; 26: 54-61
- Hardin H, Montemayor-Garcia C, Lloyd RV.?et al.?Thyroid cancer stem-like cells and epithelial-mesenchymal transition in thyroid cancers. Hum Pathol 2013; 44: 1707-13
Address for correspondence
Publication History
Article published online:
23 June 2021
? 2018. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2,
Noida-201301 UP, India

|?Figure.1Kaplan?Meir curves of disease-free recurrent in pediatric age groups and young adult age groups
References
- oltz MM, Enomoto L, Ornstein R, Saunders B, Hollenbeak C.?Incidence and survival differences of differentiated thyroid cancer among younger women. Clin Oncol Adolesc Young Adults 2013; 3: 79-88
- a Vecchia C, Malvezzi M, Bosetti C, Garavello W, Bertuccio P.?et al.?Thyroid cancer mortality and incidence: A global overview. Int J Cancer 2015; 136: 2187-95
- haukar DA, Vaidya AD. Pediatric thyroid cancers: An Indian perspective?Indian J Surg Oncol?2012; 3:?166-72
- ung W, Sarlis NJ.?Current controversies in the management of pediatric patients with well-differentiated nonmedullary thyroid cancer: A review. Thyroid 2002; 12: 683-702
- rancis GL, Waguespack SG, Bauer AJ, Angelos P, Benvenga S.?et al.?Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid 2015; 25: 716-59
- ordioli MI, Moraes L, Cury AN, Cerutti JM.?Are we really at the dawn of understanding sporadic pediatric thyroid carcinoma?. Endocr Relat Cancer 2015; 22: R311-24
- hozeimeh N, Gingalewski C. Thyroid nodules in children: A single institution's experience.J. Oncol.2011;2011: 974125. Published online 2011 Oct 6.doi: 10.1155/2011/974125
- ee YA, Jung HW, Kim HY, Choi H, Kim HY, Hah JH.?et al.?Pediatric patients with multifocal papillary thyroid cancer have higher recurrence rates than adult patients: A retrospective analysis of a large pediatric thyroid cancer cohort over 33 years. J Clin Endocrinol Metab 2015; 100: 1619-29
- ada N, Sugino K, Mimura T, Nagahama M, Kitagawa W, Shibuya H. et al.?Pediatric differentiated thyroid carcinoma in stage I: Risk factor analysis for disease free survival. 2009; 9: 306
- Chen Q, Zou XH, Wei T, Huang QS, Sun YH.?Prediction of ipsilateral and contralateral central lymph node metastasis in unilateral papillary thyroid carcinoma: A retrospective study. Gland Surg 2015; 4: 288-94
- Conzo G, Docimo G, Pasquali D, Mauriello C, Gambardella C, Esposito D.?et al.?Predictive value of nodal metastases on local recurrence in the management of differentiated thyroid cancer. Retrospective clinical study. BMC Surg 2013; 13 Suppl2: S3
- Nixon IJ, Ganly I, Patel SG, Morris LG, Palmer FL, Thomas D.?Observation of clinically negative central compartment lymph nodes in papillary thyroid carcinoma. Surgery 2013; 154: 1166-72
- Barczy?ski M, Konturek A, Stopa M, Nowak W.?Prophylactic central neck dissection for papillary thyroid cancer. Br J Surg 2013; 100: 410-8
- Giordano D, Valcavi R, Thompson GB, Pedroni C, Renna L, Gradoni P.?et al.?Complications of central neck dissection in patients with papillary thyroid carcinoma: Results of a study on 1087 patients and review of the literature. Thyroid 2012; 22: 911-7
- Chisholm EJ, Kulinskaya E, Tolley NS. Systematic review and meta-analysis of the adverse effects of thyroidectomy combined with central neck dissection as compared with thyroidectomy alone?Laryngoscope?2009; 119:?1135-9
- Givens DJ, Buchmann LO, Agarwal AM, Grimmer JF, Hunt JP. BRAF V600E does not predict aggressive features of pediatric papillary thyroid carcinoma?Laryngoscope?2014; 124:?E389-93
- Fenton CL, Lukes Y, Nicholson D, Dinauer CA, Francis GL, Tuttle RM.?The ret/PTC mutations are common in sporadic papillary thyroid carcinoma of children and young adults. J Clin Endocrinol Metab 2000; 85: 1170-5
- Chapnick DA, Warner L, Bernet J, Rao T, Liu X. Partners in crime: The TGF? and MAPK pathways in cancer progression?Cell Biosci?2011; 1:?42
- Buehler D, Hardin H, Shan W, Montemayor-Garcia C, Rush PS, Asioli S. et al.?Expression of epithelial-mesenchymal transition regulators SNAI2 and TWIST1 in thyroid carcinomas. Mod Pathol 2013; 26: 54-61
- Hardin H, Montemayor-Garcia C, Lloyd RV.?et al.?Thyroid cancer stem-like cells and epithelial-mesenchymal transition in thyroid cancers. Hum Pathol 2013; 44: 1707-13


 PDF
PDF  Views
Views  Share
Share

