Prognostic Factors and Survival Outcomes of Intracranial Ependymoma Treated with Multimodality Approach
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(04): 420-426
DOI: DOI: 10.4103/ijmpo.ijmpo_202_15
Abstract
Objectives: We aimed to analyze treatment outcomes of intracranial ependymoma (ICE) treated at our institute with multimodality approach. Materials and Methods: Demography, treatment details, and survival data of 40 patients (2005–2012) were collected in a predesigned pro forma. Kaplan Meier method was used to analyze disease-free survival (DFS) and the impact of prognostic factors was determined using univariate analysis (log-rank test). Multivariate analysis was performed using Cox-proportional hazard model. SPSS version 21.0 was used for all statistical analysis. Results: Male:female ratio was 29:11. Gross total resection: subtotal resection or less was 42.5%: 57.5%. A total of 16 patients (40%) had anaplastic histology. All except two patients received adjuvant radiotherapy. Four patients received concurrent chemotherapy (temozolomide [TMZ]) and 10 patients received adjuvant chemotherapy (6 carboplatin plus etoposide; 4 TMZ). Median follows up was 18 months (2–60 months). Median DFS for the entire cohort was 22.42 months. The estimated 1, 2, and 3 years DFS was found to be 58.5%, 41%, and 30.7%, respectively. On univariate analysis, patients receiving higher radiation dose (56 Gray vs. 60 Gray; hazard ratio [HR] 0.366; 95% confidence interval [CI] 0.142–0.9553; P = 0.02) and lower MIB labeling index (<20 class="i" xss=removed>P = 0.001) had a better DFS. Higher radiation dose continued to be an independent prognostic factor on multivariate analysis (HR 0.212; 95% CI 0.064–0.856; P = 0.03). Conclusion: ICE has guarded prognosis. Adjuvant radiotherapy to a higher radiation dose improves survival. Higher MIB labeling index connotes a dismal survival despite the use of radiotherapy and chemotherapy.
Publication History
Article published online:
04 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used forcommercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Objectives:
We aimed to analyze treatment outcomes of intracranial ependymoma (ICE) treated at our institute with multimodality approach.
Materials and Methods:
Demography, treatment details, and survival data of 40 patients (2005–2012) were collected in a predesigned pro forma. Kaplan Meier method was used to analyze disease-free survival (DFS) and the impact of prognostic factors was determined using univariate analysis (log-rank test). Multivariate analysis was performed using Cox-proportional hazard model. SPSS version 21.0 was used for all statistical analysis.
Results:
Male:female ratio was 29:11. Gross total resection: subtotal resection or less was 42.5%: 57.5%. A total of 16 patients (40%) had anaplastic histology. All except two patients received adjuvant radiotherapy. Four patients received concurrent chemotherapy (temozolomide [TMZ]) and 10 patients received adjuvant chemotherapy (6 carboplatin plus etoposide; 4 TMZ). Median follows up was 18 months (2–60 months). Median DFS for the entire cohort was 22.42 months. The estimated 1, 2, and 3 years DFS was found to be 58.5%, 41%, and 30.7%, respectively. On univariate analysis, patients receiving higher radiation dose (56 Gray vs. 60 Gray; hazard ratio [HR] 0.366; 95% confidence interval [CI] 0.142–0.9553; P = 0.02) and lower MIB labeling index (<20 xss=removed>P = 0.001) had a better DFS. Higher radiation dose continued to be an independent prognostic factor on multivariate analysis (HR 0.212; 95% CI 0.064–0.856; P = 0.03).
Conclusion:
ICE has guarded prognosis. Adjuvant radiotherapy to a higher radiation dose improves survival. Higher MIB labeling index connotes a dismal survival despite the use of radiotherapy and chemotherapy.
Introduction
Ependymoma accounts for <2 href="https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5759057/#ref1" rid="ref1" class=" bibr popnode" role="button" aria-expanded="false" aria-haspopup="true" xss=removed>1] In children, this is the 3rd most common central nervous system tumor and half of these are below 5 years of age.[2] These tumors arise from the ependymal cell lining the ventricles and central canal. Gross total excision (GTE) is considered the cornerstone of therapy.[1,2] Adjuvant radiotherapy is often delivered to improve long-term disease control. Over the years, radiation treatment for ependymoma has evolved from cranio spinal radiation (CSI)[3,4] to focal radiation.[5,6] Adjuvant chemotherapy is still investigational in the adjuvant or preradiotherapy setting.[7,8] In present contemporary series, 5 years disease-free survival (DFS) and overall survival (OS) in adult ependymal tumors have been reported to be around 40%–50% and 60%–70%, respectively.[9] For pediatric patients, 3–7 years DFS has been reported to be ranging from 30% to 61%.[6,7]
Management of ependymoma in the present era is truly multidisciplinary. Surgery plays a pivotal role and if required, second look surgery has been shown to improve survival. Adjuvant therapy in the form of conformal radiation is also not universally available or accessible to the patients in our country. Several challenges thus encompass the management in a resource constrained setting like ours. Due to these factors, the outcome in our setting might not be at par with those of the developed world. Hence, in this report, we intended to present our experience of treating patients of intracranial ependymoma (ICE) with adjuvant radiotherapy with or without chemotherapy and also identify challenges which could lead to further improvement in the outcome of ICE patients in our country.
Materials and Methods
This report includes 40 patients treated at our institute from 2005 to 2012. A total of 42 patients with confirmed histopathology were identified. Two patients defaulted after being registered, and their complete medical records were missing and were excluded from the analysis. This retrospective analysis of 40 patients was approved by our institutional review board and all the patients signed the informed consent form before initiation of treatment.
Patient and treatment related variables as documented in the file were recorded on a structured pro forma. Patient related factors analyzed were age, sex, symptoms, symptom duration, Karnofsky performance status, and treatment related factors analyzed were preoperative/operative diagnosis, extent of surgical resection, interval between surgery and start of radiotherapy, histopathological findings, details of radiotherapy treatment, details of concurrent and adjuvant chemotherapy, and toxicities during and after treatment.
All patients underwent maximal safe resection ([GTE; >90% resection], subtotal excision [STE; <90>
Radiotherapy was delivered in all patients with a three-dimensional conformal radiotherapy technique on a linear accelerator. A thermoplastic immobilization device was used for each patient for treatment position reproducibility. A contrast-enhanced computed tomography scan was done in the treatment position and the treatment volume was decided based on the preoperative MRI images. Initial clinical target volume included the enhancing tumor and edema (in case of high-grade tumors) with a 2 cm margin all around as seen in the preoperative T2-weighted/T2 flair MRI scan. Boost phase included the T1 contrast enhancing tumor volume with a 2 cm margin. For grade II tumors, 1 cm margin (given to tumor bed) was used for both initial as well as boost phase planning. A uniform expansion of 5 mm was given all around the CTV to generate the planning target volume (PTV). 56–60 Gray was delivered at 2 Gray per fraction (50 Gray in 25 fractions to initial CTV followed by a boost of 6–10 Gray in 3–5 fractions). A radiation dose of 60 Gray was used in patients with high-grade histology and in patients with STE at physician's discretion. In patients with CSF dissemination, CSI was used. For CSI, CTV included the entire brain and spinal axis extending at least 1 cm beyond the thecal sac (as determined from MRI images). 5 mm margin was given around CTV to delineate PTV. The whole cranium received a dose of 36 Gray in 20 fractions over 4 weeks followed by boost of 20 Gray in 10 fractions over 2 weeks to the posterior fossa. Dose to the spinal axis was 36 Gray at 1.8 Gray per fraction followed by a boost dose of 5.4–9 Gray at 1.8 Gray per fraction (for isolated spinal drop metastasis). Planning was done using Eclipse treatment planning system Version 6.5 (Varian Medical Systems, Palo Alto, CA, USA) and patients were treated on a linear accelerator, CL 2300 CD (Varian Medical System, Palo Alto, California, United States)
Adjuvant chemotherapy (indication as well as regimen) was used at physician's discretion in patients with STE. The chemotherapy schedule consisted of 6 cycles of carboplatin and etoposide (CE) (injection carboplatin area under curve 5 on day 1 plus injection etoposide 100 mg/m2 on day 1–3 repeated every 3 weeks). Patients with CSF dissemination or spinal drop metastasis received injection vincristine (1.4 mg/m2; maximum 2 mg intravenous weekly) and intrathecal methotrexate (15 mg once a week until three consecutive CSF were negative for tumor cells) in addition to the standard chemotherapy. Patients aging <3 xss=removed>2 daily and adjuvant TMZ was started after a gap of 1 month. The first cycle was given at 150 mg/m2 (day 1–5) and depending on the tolerance increased to 200 mg/m2 in the next cycle for a minimum of six cycles every 4 weeks.
Complete blood count, liver function test, and renal function test was repeated once a week during radiation and before each cycle of adjuvant chemotherapy. Toxicities were evaluated by common terminology criteria for adverse events version 2.0 (National Cancer Institute, USA). Patients presenting with features of raised intracranial tension or any grade 3 or higher hematological or nonhematological toxicities were managed indoors with intravenous antibiotics, growth factors, transfusion of blood products, and supportive care as required.
After completion of treatment, patients were evaluated with periodic clinical and radiological examination. Patients were followed 1 month after completion of radiation and subsequently every 3 months for first 2 years, every 6 months for next 3 years, and yearly thereafter. A contrast-enhanced MRI of the brain and spine was ordered, starting from second follow up visit and repeated subsequently every 6 months till 5 years and yearly thereafter. Response evaluation was done by Mac Donald's criteria.[10]
The recurrences were worked up with contrast-enhanced MRI of brain and spine as well as CSF cytology. For a localized recurrence, surgical salvage was considered followed by consolidation with re-irradiation or chemotherapy. In patients with a disseminated recurrence, chemotherapy alone was considered. The chemotherapy schedule for salvage consisted of VEC (injection vincristine 1.5 mg/m2 [maximum 2 mg], injection etoposide 100 mg/m2 [intravenous day 1–3], and injection carboplatin area under curve 5 [intravenous day 1]) with or without intrathecal methotrexate.
DFS was calculated from the date of surgery till the date of documented disease progression or death and Kaplan Meier method was used for survival analysis. Univariate analysis (log rank test) was used to assess the impact of age (<!--≥20 years), extent of surgery, radiotherapy dose (56 Gray vs. 60 Gray), grade of tumor (grade II vs. III), MIB labeling index (</≥20), and adjuvant chemotherapy on DFS. Multivariate analysis was performed using Cox-proportional hazard model.
Results
Patient characteristics are summarized in Table 1. The median time interval between surgery and initiation of radiotherapy was 36 days (range: 28–70 days). All patients except two (one grade II histology patient preferred follow up denying radiation and one patient progressed during preradiation chemotherapy schedule) received radiotherapy. Median radiotherapy dose delivered was 56 Gray (45–60 Gray). Craniospinal irradiation was delivered in 5 patients. Dose to the craniospinal axis was 36 Gray and the local cranial dose was 56 Gray. The compliance to radiation was excellent, and all patients completed the stipulated treatment. The median duration of radiotherapy treatment was 50 days (range 42–58 days).
Table 1
Patient characteristics
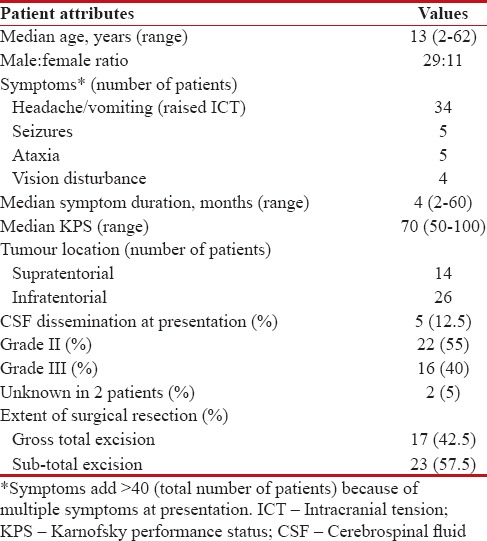
|
Four patients received concurrent and adjuvant TMZ and 6 patients received adjuvant chemotherapy with CE regimen. Median number of chemotherapy cycles was 6 (range-3–6). 4 (10%) patients developed grade III or higher hematological toxicity and no patients developed febrile neutropenia. One patient developed Grade 2 gastrointestinal toxicity and 1 patient developed Grade 3 gastrointestinal toxicity. Grade I skin toxicity was seen in 5 patients and 1 patient developed grade II skin toxicity during radiotherapy.
Median follow up was 18 months (2–60 months). At the time of the last follow-up, 16 patients were found to be disease-free, 18 had progression, and 6 were lost to follow up (these 6 patients were excluded from all survival analysis). Median DFS for the entire cohort was 22.42 months. The estimated 1, 2, and 3 year DFS was found to be 58.5%, 41%, and 30.7%, respectively [Figure 1]. On univariate analysis, patients receiving higher radiation dose (56 Gray vs. 60 Gray; hazard ratio [HR] 0.366; 95% confidence interval [CI] 0.142–0.9553; P = 0.02) and lower MIB labeling index (<20 xss=removed>P = 0.001) had a better DFS [Figures [Figures2,2, ,3,3, and Table 2]. Higher radiation dose continued to be an independent prognostic factor on multivariate analysis (HR 0.212; 95% CI 0.064–0.856; P = 0.03). Other prognostic factors retained on multivariate analysis did not show the statistically significant impact on DFS.
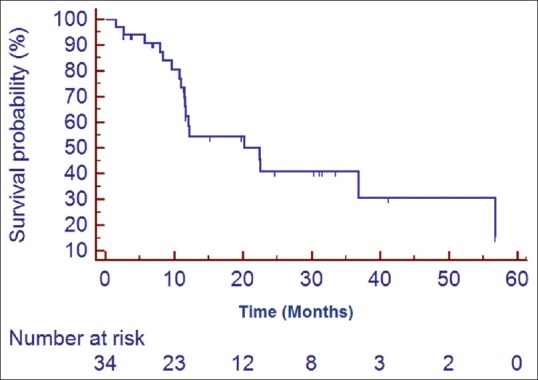
| Figure 1:Kaplan Meier estimates of disease free survival of the entire cohort
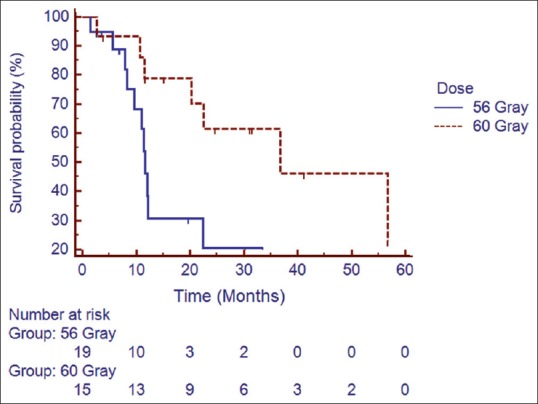
| Figure 2:Impact of radiation dose on disease free survival
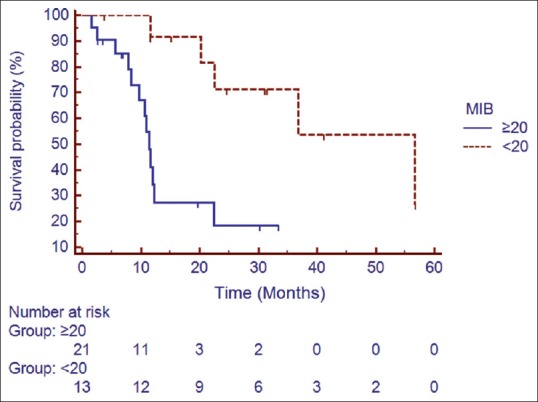
| Figure 3:Impact of MIB labelling index on disease free survival
Table 2
Impact of prognostic variables on disease free survival
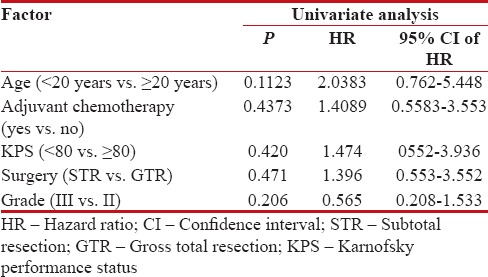
| The most common site of failure was tumor bed (14/18 patients). Three patients had elsewhere brain failures, and 2 patients had spinal deposits (one had both tumor bed and spinal recurrence). Surgical excision of a recurrent tumor was done in 7 patients of whom 2 also received postoperative radiotherapy. One patient underwent re-irradiation alone and two received salvage chemotherapy. Seven patients did not receive any treatment at progression. Of the 10 patients receiving treatment for recurrent disease, 3 patients were disease-free at the time of last follow up.
Discussion
Ependymoma is a glial tumor arising from ependymal cells in the periventricular area. Percival Bailey, in 1924 coined the term ependymoma for a group of tumors arising from ependymal cells.[11] Although ependymoma (like germ cell tumors) have a propensity for CSF dissemination, the exact frequency is debated in the existing literature (9%–20%). This figure was 12.5% in our patient cohort. The WHO has classified ependymoma in three distinct groups. Myxopapillary and subependymal tumors are classified as grade I. Grade II ependymoma are the most common variant of ependymoma followed by anaplastic ependymoma or grade III ependymoma. Grade II ependymoma has four distinct types viz., cellular, papillary, clear cells, tynactic.[1] The present analysis only included grade II and III tumors; 57.5% of patients in our series had grade II histology.
Ependymoma most commonly arise from the posterior fossa (60%–70%). Due to the primary tumor location these tumors often present with acute symptoms secondary to raised intracranial tension. However, supratentorial ependymoma is often associated with mood changes, personality changes, and lobar syndromes. Nearly 65% of patients in our cohort had infratentorial tumor location and 85% of patients had symptoms of raised intracranial tension.
Surgical resection has long been considered the most important treatment for ependymoma. A GTE has been reported to impart survival advantage[6,7] and is often attempted if feasible without increasing morbidity. In general, GTE can be accomplished in 50%–79% of patients. Some authors advocate second look surgery whenever possible to optimize survival. However, the decision is crucial and needs to be weighed against potential complications. In a study of 45 patients, Rogers et al.[12] reported 10 years OS rates of 83% versus 43% in patients treated with GTE plus radiotherapy versus STE plus radiotherapy. GTE was also associated with a lower risk of death from any cause (HR: 0·16, 95% CI: 0·07–0·37, P ≤ 0·0001) compared with near- or subtotal resection in the study by Merchant et al.[6] two years DFS for patients with GTE versus STE in our study was 55% versus 30%, respectively. However, the difference was not statistically significant. Only 42.5% of patients in the study could undergo GTE.
Radiotherapy is considered the most important adjuvant therapy to optimize long-term disease control.[13] The philosophy of radiation treatment has been rapidly evolving over the period. While earlier series[3,4] recommended larger fields (whole brain for supratentorial, whole brain with cervical cord extension for infratentorial and CSI for high-grade ependymoma), more recent series have shown the patterns of failure to be predominantly local.[5] Only 2/9 patients receiving tumor bed plus margin had a recurrence in the study by Paulino et al. and both of them occurred in the tumor bed itself. In a large series[6] of 153 patients, authors used 1 cm margin only to the tumor bed as the target volume and reported all local failures (21 patients) to lie within the 95% isodose volume. Current Children's Oncology Group (Protocol) is using a further reduced volume of tumor bed plus 0.5 cm margin for CTV. We used tumor bed plus 2 cm margin in our study, and most of the failures in our study were also in tumor bed itself (13/17 failures).
The dose response has always remained an area of active research in ependymoma. Conventionally, a dose of 50 Gray is considered adequate for low grade and 60 Gray for high-grade tumors, respectively. Paulino, however, failed to establish a dose response relationship beyond 45 Gray.[5] Merchant et al.[6] reported improved outcome in patients with anaplastic ependymoma when treated with a higher dose of radiation. Review of older series revealed better survival with a radiation dose of 45 Gray or higher. Recently, studies are evaluating a further dose escalation of >54 Gray. Patients receiving a higher dose in our series did significantly better than those treated with a lower dose (P = 0.0229). Two years DFS was 68% versus 21% in favor of the higher dose. In an interesting prospective study, Massimino et al. randomized children to either hyperfractionated radiotherapy (HFRT) 70.4 Gy (1.1 Gy/fraction twice daily) or conventionally fractionated radiotherapy to improve disease control by increasing the total dose of radiation but failed to show improved outcome with HFRT.[14]
Adjuvant chemotherapy has long been hypothesized to improve survival in ependymoma. However, there exists little data to support the use of chemotherapy. In few reports, authors have reported variable rate of chemo sensitivity. The response to a single agent and multi agent chemotherapy has ranged from 0% to 11% and 0%–86%, respectively. White et al.[15] reported 86% response rate in 7 patients (<4 xss=removed>et al.[16] reported 48% response in 25 patients younger than 3 years when treated with 2 cycles of vincristine and cyclophosphamide. The HIT trial[7] reported 3 years OS to be 75.6% with adjuvant chemotherapy administered after completion of radiotherapy. We could not find any effect of adjuvant chemotherapy on survival in our series (P = 0.48).
Recurrences happen in approximately 50% of patients of ependymoma. The median time to recurrence is 13–25 months.[17,18] Recurrences are predominantly local and only 20% have isolated distant recurrence. The treatment options are limited after a recurrence. Surgical excision of recurrent disease should be tried in all cases followed by postoperative radiotherapy or due to consideration given to re-irradiation.[19,20] Reirradiation has been found to yield durable response at times. Bouffet et al.[19] treated 18 of 47 recurrent ependymoma patients with 54 Gray reirradiation and reported 3 years OS of 81%. Although there were no severe acute toxicities, 2/18 patients had endocrine dysfunction later on and 1 patient required special educational support. Stereotactic radiotherapy is often considered as an effective modality to minimize toxicity during re-irradiation.[21] In the recent years, proton therapy has also emerged as an effective option to treat recurrent ependymoma without increasing morbidity.[22,23] However, there are limited prospective data on these promising modalities.
Salvage chemotherapy for recurrent or metastatic patients has been found to be of questionable benefit. The response rates for single or combination chemotherapy has been found to be 12.9% and 17.4%, respectively.[24] Concerns of toxicities have been keeping physicians away from reirradiation, however in view of encouraging results reported by Bouffet et al.[19] and Merchant et al.,[20] it appears imperative to use this modality to achieve a durable response. Only 1 patient received reirradiation in our cohort and 2 patients received postoperative radiotherapy.
Great enthusiasm has been witnessed in classifying ependymoma in different distinct molecular subgroups to optimize treatment and outcome. Two landmark articles by Witt et al.[25] and Pajtler et al.[26] classified ependymoma in distinct molecular and prognostic subgroup. This classification makes it is possible to deliver treatment directed to the distinct molecular characteristic.[27] However, the cost of such classification is a major limitation to include in regular patient care and needs prospective validation.
Retrospective nature of the present study is a limitation in itself. Our patients also had less rates of GTE and outcomes are not comparable to those of developed world series. Relatively, short follow up and lost to follow up are other limitations of our present study. The effectiveness of this combined modality approach in an unselected patient population outside a clinical trial (simulating a real world scenario) in a resource constrained setting can be considered as the strength of the data.
Several challenges exist in the optimal management of brain tumors including ependymoma in our country. Most of the patients whom we treat are referred from low volume centers where neurosurgical facilities are less developed and a second look surgery is not done routinely because of long waiting times or resource constraints. Radiation facilities (particularly conformal radiation and facilities of radiation for pediatric patients, many of them requiring general anesthesia) are not universally available and accessible to all patients, and few centers include ours provide these treatments. This “double trouble” leads ultimately in patients receiving either sub-optimal surgery or radiation or both which leads to an inferior outcome. These tumors also are predominant in the pediatric population and require long-term follow up not only for surveillance of disease recurrence but also for neurocognitive outcomes. Since patients come to our center from far off places, many of them are lost to follow up immediately after or within 2–3 years of completion of treatment. This further precludes a better analysis of prognostic factors as well as survival.
Ours is the largest series published till date from India and could serve as a benchmark and comparator for other studies too. The challenges and the factors identified in our study leading to inferior outcomes may help other centers in overcoming these hurdles prospectively and thus leading to improvement in the outcome of patients with ICE in our country.
Conclusion
ICE is a rare group of malignancies. Maximal safe resection followed by post-operative radiotherapy to a higher dose of 60 Gray (particularly for anaplastic ependymoma) should be used. Higher MIB labeling index confers poor prognosis. Role of chemotherapy remains controversial, and radiation therapy should not be delayed even in young patients. Reirradiation and surgical excision with postoperative radiation therapy remain valid treatment options at recurrence and may yield durable response in some patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Gondi V, Vogelbaum MA, Grimm S, Mehta MP. Primary intracranial neoplasm. In: Halperin EC, Wazer DE, Perez CA, Brady LW, editors. Perez and Brady's Principles and Practice of Radiation Oncology. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2013. p. 669-70.
- Freeman CR, Farmer JP, Taylor RE. Central Nervous System Tumors in Children. In: Halperin EC, Wazer DE, Perez CA, Brady LW, editors. Perez and Brady's Principles and Practice of Radiation Oncology. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2013. p. 1643-5.
- Salazar OM, Castro-Vita H, VanHoutte P, Rubin P, Aygun C. Improved survival in cases of intracranial ependymoma after radiation therapy. Late report and recommendations. J Neurosurg 1983;59:652-9.
- Goldwein JW, Corn BW, Finlay JL, Packer RJ, Rorke LB, Schut L. Is craniospinal irradiation required to cure children with malignant (anaplastic) intracranial ependymomas? Cancer 1991;67:2766-71.
- Paulino AC. The local field in infratentorial ependymoma: Does the entire posterior fossa need to be treated? Int J Radiat Oncol Biol Phys 2001;49:757-61.
- Merchant TE, Li C, Xiong X, Kun LE, Boop FA, Sanford RA. Conformal radiotherapy after surgery for paediatric ependymoma: A prospective study. Lancet Oncol 2009;10:258-66.
- Timmermann B, Kortmann RD, Kühl J, Rutkowski S, Dieckmann K, Meisner C, et al. Role of radiotherapy in anaplastic ependymoma in children under age of 3 years: Results of the prospective German brain tumor trials HIT-SKK 87 and 92. Radiother Oncol 2005;77:278-85.
- Needle MN, Goldwein JW, Grass J, Cnaan A, Bergman I, Molloy P, et al. Adjuvant chemotherapy for the treatment of intracranial ependymoma of childhood. Cancer 1997;80:341-7.
- Reni M, Brandes AA, Vavassori V, Cavallo G, Casagrande F, Vastola F, et al. Amulticenter study of the prognosis and treatment of adult brain ependymal tumors. Cancer 2004;100:1221-9.
- Macdonald DR, Cascino TL, Schold SC Jr., Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 1990;8:1277-80.
- Bailey P. A study of tumors arising from ependymal cells. Arch Neurol Psychiatry 1924;11:1-27.
- Rogers L, Pueschel J, Spetzler R, Shapiro W, Coons S, Thomas T, et al. Is gross-total resection sufficient treatment for posterior fossa ependymomas? J Neurosurg 2005;102:629-36.
- Rousseau P, Habrand JL, Sarrazin D, Kalifa C, Terrier-Lacombe MJ, Rekacewicz C, et al. Treatment of intracranial ependymomas of children: Review of a 15-year experience. Int J Radiat Oncol Biol Phys 1994;28:381-6.
- Massimino M, Gandola L, Giangaspero F, Sandri A, Valagussa P, Perilongo G, et al. Hyperfractionated radiotherapy and chemotherapy for childhood ependymoma: Final results of the first prospective AIEOP (Associazione Italiana di Ematologia-Oncologia Pediatrica) study. Int J Radiat Oncol Biol Phys 2004;58:1336-45.
- White L, Kellie S, Gray E, Toogood I, Waters K, Lockwood L, et al. Postoperative chemotherapy in children less than 4 years of age with malignant brain tumors: Promising initial response to a VETOPEC-based regimen. A Study of the Australian and New Zealand Children's Cancer Study Group (ANZCCSG). J Pediatr Hematol Oncol 1998;20:125-30.
- Duffner PK, Krischer JP, Sanford RA, Horowitz ME, Burger PC, Cohen ME, et al. Prognostic factors in infants and very young children with intracranial ependymomas. Pediatr Neurosurg 1998;28:215-22.
- Bouffet E, Perilongo G, Canete A, Massimino M. Intracranial ependymomas in children: A critical review of prognostic factors and a plea for cooperation. Med Pediatr Oncol 1998;30:319-29.
- Foreman NK, Love S, Gill SS, Coakham HB. Second-look surgery for incompletely resected fourth ventricle ependymomas: Technical case report. Neurosurgery 1997;40:856-60.
- Bouffet E, Hawkins CE, Ballourah W, Taylor MD, Bartels UK, Schoenhoff N, et al. Survival benefit for pediatric patients with recurrent ependymoma treated with reirradiation. Int J Radiat Oncol Biol Phys 2012;83:1541-8.
- Merchant TE, Boop FA, Kun LE, Sanford RA. A retrospective study of surgery and reirradiation for recurrent ependymoma. Int J Radiat Oncol Biol Phys 2008;71:87-97.
- Murai T, Sato K, Iwabuchi M, Manabe Y, Ogino H, Iwata H, et al. Re-irradiation of recurrent anaplastic ependymoma using radiosurgery or fractionated stereotactic radiotherapy. Jpn J Radiol 2016;34:211-8.
- Ares C, Albertini F, Frei-Welte M, Bolsi A, Grotzer MA, Goitein G, et al. Pencil beam scanning proton therapy for pediatric intracranial ependymoma. J Neurooncol 2016;128:137-45.
- Eaton BR, Chowdhry V, Weaver K, Liu L, Ebb D, MacDonald SM, et al. Use of proton therapy for re-irradiation in pediatric intracranial ependymoma. Radiother Oncol 2015;116:301-8.
- Bouffet E, Capra M, Bartels U. Salvage chemotherapy for metastatic and recurrent ependymoma of childhood. Childs Nerv Syst 2009;25:1293-301.
- Witt H, Mack SC, Ryzhova M, Bender S, Sill M, Isserlin R, et al. Delineation of two clinically and molecularly distinct subgroups of posterior fossa ependymoma. Cancer Cell 2011;20:143-57.
- Pajtler KW, Witt H, Sill M, Jones DT, Hovestadt V, Kratochwil F, et al. Molecular classification of ependymal tumors across all CNScompartments, histopathological grades, and age groups. Cancer Cell 2015;27:728-43.
- Benson R, Mallick S, Julka PK, Rath GK. Molecular predictive and prognostic factors in ependymoma. Neurol India 2016;64:279-86.

| Figure 1:Kaplan Meier estimates of disease free survival of the entire cohort

| Figure 2:Impact of radiation dose on disease free survival

| Figure 3:Impact of MIB labelling index on disease free survival
References
- Gondi V, Vogelbaum MA, Grimm S, Mehta MP. Primary intracranial neoplasm. In: Halperin EC, Wazer DE, Perez CA, Brady LW, editors. Perez and Brady's Principles and Practice of Radiation Oncology. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2013. p. 669-70.
- Freeman CR, Farmer JP, Taylor RE. Central Nervous System Tumors in Children. In: Halperin EC, Wazer DE, Perez CA, Brady LW, editors. Perez and Brady's Principles and Practice of Radiation Oncology. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2013. p. 1643-5.
- Salazar OM, Castro-Vita H, VanHoutte P, Rubin P, Aygun C. Improved survival in cases of intracranial ependymoma after radiation therapy. Late report and recommendations. J Neurosurg 1983;59:652-9.
- Goldwein JW, Corn BW, Finlay JL, Packer RJ, Rorke LB, Schut L. Is craniospinal irradiation required to cure children with malignant (anaplastic) intracranial ependymomas? Cancer 1991;67:2766-71.
- Paulino AC. The local field in infratentorial ependymoma: Does the entire posterior fossa need to be treated? Int J Radiat Oncol Biol Phys 2001;49:757-61.
- Merchant TE, Li C, Xiong X, Kun LE, Boop FA, Sanford RA. Conformal radiotherapy after surgery for paediatric ependymoma: A prospective study. Lancet Oncol 2009;10:258-66.
- Timmermann B, Kortmann RD, Kühl J, Rutkowski S, Dieckmann K, Meisner C, et al. Role of radiotherapy in anaplastic ependymoma in children under age of 3 years: Results of the prospective German brain tumor trials HIT-SKK 87 and 92. Radiother Oncol 2005;77:278-85.
- Needle MN, Goldwein JW, Grass J, Cnaan A, Bergman I, Molloy P, et al. Adjuvant chemotherapy for the treatment of intracranial ependymoma of childhood. Cancer 1997;80:341-7.
- Reni M, Brandes AA, Vavassori V, Cavallo G, Casagrande F, Vastola F, et al. Amulticenter study of the prognosis and treatment of adult brain ependymal tumors. Cancer 2004;100:1221-9.
- Macdonald DR, Cascino TL, Schold SC Jr., Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 1990;8:1277-80.
- Bailey P. A study of tumors arising from ependymal cells. Arch Neurol Psychiatry 1924;11:1-27.
- Rogers L, Pueschel J, Spetzler R, Shapiro W, Coons S, Thomas T, et al. Is gross-total resection sufficient treatment for posterior fossa ependymomas? J Neurosurg 2005;102:629-36.
- Rousseau P, Habrand JL, Sarrazin D, Kalifa C, Terrier-Lacombe MJ, Rekacewicz C, et al. Treatment of intracranial ependymomas of children: Review of a 15-year experience. Int J Radiat Oncol Biol Phys 1994;28:381-6.
- Massimino M, Gandola L, Giangaspero F, Sandri A, Valagussa P, Perilongo G, et al. Hyperfractionated radiotherapy and chemotherapy for childhood ependymoma: Final results of the first prospective AIEOP (Associazione Italiana di Ematologia-Oncologia Pediatrica) study. Int J Radiat Oncol Biol Phys 2004;58:1336-45.
- White L, Kellie S, Gray E, Toogood I, Waters K, Lockwood L, et al. Postoperative chemotherapy in children less than 4 years of age with malignant brain tumors: Promising initial response to a VETOPEC-based regimen. A Study of the Australian and New Zealand Children's Cancer Study Group (ANZCCSG). J Pediatr Hematol Oncol 1998;20:125-30.
- Duffner PK, Krischer JP, Sanford RA, Horowitz ME, Burger PC, Cohen ME, et al. Prognostic factors in infants and very young children with intracranial ependymomas. Pediatr Neurosurg 1998;28:215-22.
- Bouffet E, Perilongo G, Canete A, Massimino M. Intracranial ependymomas in children: A critical review of prognostic factors and a plea for cooperation. Med Pediatr Oncol 1998;30:319-29.
- Foreman NK, Love S, Gill SS, Coakham HB. Second-look surgery for incompletely resected fourth ventricle ependymomas: Technical case report. Neurosurgery 1997;40:856-60.
- Bouffet E, Hawkins CE, Ballourah W, Taylor MD, Bartels UK, Schoenhoff N, et al. Survival benefit for pediatric patients with recurrent ependymoma treated with reirradiation. Int J Radiat Oncol Biol Phys 2012;83:1541-8.
- Merchant TE, Boop FA, Kun LE, Sanford RA. A retrospective study of surgery and reirradiation for recurrent ependymoma. Int J Radiat Oncol Biol Phys 2008;71:87-97.
- Murai T, Sato K, Iwabuchi M, Manabe Y, Ogino H, Iwata H, et al. Re-irradiation of recurrent anaplastic ependymoma using radiosurgery or fractionated stereotactic radiotherapy. Jpn J Radiol 2016;34:211-8.
- Ares C, Albertini F, Frei-Welte M, Bolsi A, Grotzer MA, Goitein G, et al. Pencil beam scanning proton therapy for pediatric intracranial ependymoma. J Neurooncol 2016;128:137-45.
- Eaton BR, Chowdhry V, Weaver K, Liu L, Ebb D, MacDonald SM, et al. Use of proton therapy for re-irradiation in pediatric intracranial ependymoma. Radiother Oncol 2015;116:301-8.
- Bouffet E, Capra M, Bartels U. Salvage chemotherapy for metastatic and recurrent ependymoma of childhood. Childs Nerv Syst 2009;25:1293-301.
- Witt H, Mack SC, Ryzhova M, Bender S, Sill M, Isserlin R, et al. Delineation of two clinically and molecularly distinct subgroups of posterior fossa ependymoma. Cancer Cell 2011;20:143-57.
- Pajtler KW, Witt H, Sill M, Jones DT, Hovestadt V, Kratochwil F, et al. Molecular classification of ependymal tumors across all CNScompartments, histopathological grades, and age groups. Cancer Cell 2015;27:728-43.
- Benson R, Mallick S, Julka PK, Rath GK. Molecular predictive and prognostic factors in ependymoma. Neurol India 2016;64:279-86.


 PDF
PDF  Views
Views  Share
Share

