Recurrent Atypical Meningioma with Pleural Metastases
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2018; 39(02): 241-243
DOI: DOI: 10.4103/ijmpo.ijmpo_98_17
Abstract
Meningiomas are the most common primary intracranial tumors in adults. Although Grade I meningiomas are considered benign, Grade II/III (atypical and anaplastic) meningiomas are known to be locally aggressive, recurrent, and rarely present with distant metastases. We report a 40-year-old female with recurrent atypical meningioma (WHO Grade II) who presented with features suggestive of a massive right-sided pleural effusion. Imaging showed bilateral large pleural-based lesions, and histopathological examination and immunohistochemistry of the mass were consistent with metastatic atypical meningioma. A high index of suspicion is warranted to detect extracranial metastases, especially in patients with recurrent meningiomas and higher WHO grade of tumor.
Publication History
Article published online:
23 June 2021
© 2018. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Meningiomas are the most common primary intracranial tumors in adults. Although Grade I meningiomas are considered benign, Grade II/III (atypical and anaplastic) meningiomas are known to be locally aggressive, recurrent, and rarely present with distant metastases. We report a 40-year-old female with recurrent atypical meningioma (WHO Grade II) who presented with features suggestive of a massive right-sided pleural effusion. Imaging showed bilateral large pleural-based lesions, and histopathological examination and immunohistochemistry of the mass were consistent with metastatic atypical meningioma. A high index of suspicion is warranted to detect extracranial metastases, especially in patients with recurrent meningiomas and higher WHO grade of tumor.
Introduction
Meningiomas are one of the most common primary brain tumors accounting for 33.8% of all central nervous system (CNS) tumors.[1] Malignant meningiomas are, however, uncommon and constitute a small proportion (5% or less) of all cases. Recurrence rates of around 3% for benign meningiomas and 38% for atypical meningiomas have been reported.[2] These atypical (WHO Grade II) or anaplastic (WHO Grade III) meningiomas are likely to be locally aggressive and may very rarely present with distant extracranial metastases. Metastatic lesions have been described most frequently in the lung, bones, intraspinally, and in the liver.[3] Metastases to the pleura have been reported as early as 1944 but are infrequent.[4]
Case Report
A 40-year-old female presented with complaints of cough with scanty sputum, breathlessness at rest which improved on lying on the right side, and low-grade fever of 2 weeks' duration. She also reported significant loss of weight and appetite over the past 2 months. The patient had undergone craniotomies twice during the past 18 months for excision of recurrent meningiomas. In May 2015, she had presented with headache and left-sided hemiparesis. Magnetic resonance imaging (MRI) had shown a right frontal parasagittal meningioma, for which a craniotomy was performed with gross total excision of the tumor, following which she improved symptomatically. Histopathology of the tumor was reported as atypical meningioma (WHO Grade II) [Figure 1]a,[Figure 1]b,[Figure 1]c,[Figure 1]d,[Figure 1]e. In December 2015, 7 months following the first surgery, she developed headache and vomiting with worsening of her left hemiparesis. MRI revealed recurrence of the tumor, and she underwent another craniotomy. Intraoperatively, the tumor was found to be locally invasive and was only partially removed to minimize morbidity. Histopathological examination (HPE) of the second surgical specimens also demonstrated atypical meningioma – WHO Grade II with clear cell change. In view of incomplete resection during the second craniotomy, the patient had received adjuvant radiation therapy (30 fractions, total dose of 56 Gy) elsewhere. After being relatively symptom free for a year, she presented with respiratory symptoms in March 2017. She is a known diabetic for 3 years with fairly controlled blood sugars. There was no significant family history of malignancy.
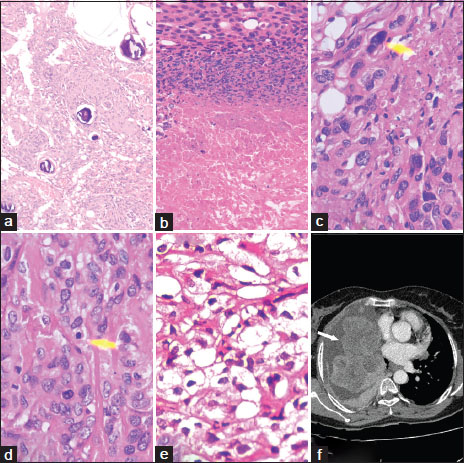
| Figure.1(a) First meningeal biopsy (H and E, ×100), (b) necrosis in tumor (H and E, ×200), (c) atypical nucleus (H and E, ×400), (d) mitotic figure (H and E, ×400), (e) clear cell change in tumor cells (H and E, ×400), (f) contrast‑enhanced computed tomography of the chest showing multiple bilateral large heterogeneous lobulated pleural‑based contrast‑enhancing lesions with associated pleural effusion
On examination, the patient was conscious, dyspneic, and pale. She was afebrile, tachycardic, and normotensive. Her oxygen saturation was 92% while she was breathing room air. Examination of the respiratory system showed features consistent with a right-sided pleural effusion. CNS examination demonstrated mild residual hemiparesis on the left side.
Blood investigations revealed anemia (Hb 8.6 g/dL), albumin/globulin reversal (2.4/3.8 g/dL), and mildly elevated erythrocyte sedimentation rate (42 mm in 1st h). Chest radiograph was suggestive of a massive loculated right pleural effusion. Diagnostic thoracocentesis was done which yielded hemorrhagic pleural fluid. Biochemical analysis showed an exudative pleural effusion (glucose 74 mg/dL, protein 5.1 g/dL, albumin 2.7 g/dL, lactate dehydrogenase 2709 U/L, adenosine deaminase of 14.96 IU/L) and cytology was negative for malignant cells. Ultrasound of the abdomen showed normal study of visualized organs. Skeletal survey was unremarkable. A contrast-enhanced computed tomography (CT) scan of the thorax was performed [Figure 1]f which revealed multiple bilateral large heterogeneous enhancing lesions which were pleural based and lobulated, with associated pleural effusion. There was no involvement of the lung parenchyma. Differential diagnoses considered were metastatic atypical meningioma, mesothelioma, or neuroendocrine tumors. The patient underwent a CT-guided biopsy of the pleural mass lesion.
HPE of the tissue revealed infiltration of the pleura by cells having indistinct cytoplasm, arranged in the form of nests, cords, and trabeculae. Some of these cells had abundant clear cytoplasm, vesicular nuclei, and 1–3 prominent nucleoli; some others had fibroblastic morphology with dark condensed nuclei and inconspicuous nucleoli. A focus of necrosis, hemorrhage, and many areas of fibrosis was seen. The tumor cells were positive for epithelial membrane antigen (UltraVision LP, Clone: E29) and vimentin (UltraVision LP, Clone: V9) but negative for mesothelin (UltraVision LP, Clone: HBME1), CK 5/6 (UltraVision LP, Clone: D5/16 B4), chromogranin (UltraVision LP, Clone: SP12), and CD 34 (UltraVision LP, Clone: QBEnd/10) [Figure 2]a,[Figure 2]b,[Figure 2]c,[Figure 2]d,[Figure 2]e,[Figure 2]f. Review of the HPE slides from the prior two surgeries showed that they closely matched the histopathology of the pleural mass. Thus, the patient was diagnosed to have recurrent atypical meningioma WHO Grade II, with pleural metastases. In view of advanced disease, the patient and her relatives opted for palliative care.{Figure 2}
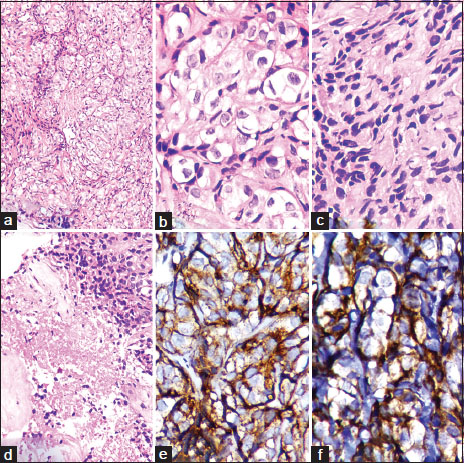
| Figure.2(a) Metastatic tumor in the pleural biopsy (H and E, ×200), (b) clear cells in the pleural metastasis (H and E, ×400), (c) fibroblastic cells in pleural metastasis (H and E, ×400), (d) necrosis in the metastatic tumor (H and E, ×200), (e) epithelial membrane antigen positivity in the tumor cells in pleural metastasis (×400), (f) vimentin positivity in the tumor cells in pleural metastasis (×400)
Discussion
Meningiomas are traditionally considered to be benign tumors; however, some are more aggressive and tend to be locally invasive. Extracranial metastases from meningiomas are extremely rare and may be found in the lungs, liver, long bones, pelvis and skull, cervical lymph nodes, pleura, vertebrae, and mediastinum. According to the WHO classification of CNS tumors (2016), meningiomas have been divided into three grades based on their histology as Grade I (meningioma), Grade II (atypical meningioma), and Grade III (anaplastic meningioma).
Atypical meningiomas
Atypical meningioma can be diagnosed on the basis of 3 of 5 histological features: spontaneous necrosis, sheeting (loss of whorling or fascicular architecture), prominent nucleoli, high cellularity, and small cells (tumor clusters with high nuclear: cytoplasmic ratio). Further brain invasion has been included as a criterion for the diagnosis of atypical meningioma.[5] Atypical and anaplastic meningiomas are notable for their high recurrence rate and the occurrence of extracranial metastasis.
Metastasis in meningiomas
Distant metastases are uncommon with benign meningiomas, but up to 5% of atypical and 30% of anaplastic meningiomas can metastasize. Meningiomas may metastasize through lymphatic, hematogenous, or cerebrospinal fluid.[6] The various sites of metastases and the reported incidences are represented in [Table 1].[3],[4] Lung metastases seem to be the most frequently reported, and pleural involvement has been documented by a few authors [Table 2].[4],[6],[7],[8],[9],[10],[11] Isolated pleural metastasis without lung involvement is rare and has been reported in a patient with frontal atypical meningioma by Yacoub et al.[11] Due to the rarity of occurrence, prognosis of metastatic meningiomas is unknown, and no definitive therapeutic regimen has been established for such cases.
|
Author, year |
Sites of metastases |
Reported incidence (%) |
|---|---|---|
|
Karasick,1974 |
Lung |
60 |
|
Abdominal viscera |
34 |
|
|
Bones (long bones, pelvis, skull, vertebrae) |
11 |
|
|
Mediastinal metastases |
18 |
|
|
Pleural involvement |
9 |
|
|
Cervical lymph nodes |
14 |
|
|
Estani slau,2009 |
Lung |
60 |
|
Abdominal viscera |
34 |
|
|
Bones (long bones, pelvis, skull, vertebrae) |
11 |
|
|
Mediastinal metastases |
5 |
|
|
Pleural involvement |
9 |
|
|
Cervical lymph nodes |
18 |
|
|
Surov,2013 |
Lung |
37.2 |
|
Bones |
16.5 |
|
|
Intraspinally |
15.2 |
|
|
Liver |
9.2 |
|
Author, year |
Primary tumor |
|---|---|
|
Russel and Sachs, 1942 |
Right parietal meningiosarcoma |
|
Dublin, 1944 |
Right parietooccipital meningiosarcoma |
|
Cross and Cooper, 1952 |
Left parietal meningioma |
|
Shuangshoti, 1970 |
Occipital angioblastic meningioma |
|
Miller and Ramsden, 1972 |
Right frontal meningioma |
|
Som, 1987 |
Parasagittal meningioma |
|
Kros, 2000 |
Papillary meningioma |
|
Kaminski, 2001 |
Frontal meningioma |
|
Yacoub, 2003 |
Frontal meningioma |
|
Emran, 2005 |
Frontal parasagittal meningioma |
|
Nakayama, 2013 |
Right parietal meningothelial meningioma |

| Figure.1(a) First meningeal biopsy (H and E, ×100), (b) necrosis in tumor (H and E, ×200), (c) atypical nucleus (H and E, ×400), (d) mitotic figure (H and E, ×400), (e) clear cell change in tumor cells (H and E, ×400), (f) contrast‑enhanced computed tomography of the chest showing multiple bilateral large heterogeneous lobulated pleural‑based contrast‑enhancing lesions with associated pleural effusion

| Figure.2(a) Metastatic tumor in the pleural biopsy (H and E, ×200), (b) clear cells in the pleural metastasis (H and E, ×400), (c) fibroblastic cells in pleural metastasis (H and E, ×400), (d) necrosis in the metastatic tumor (H and E, ×200), (e) epithelial membrane antigen positivity in the tumor cells in pleural metastasis (×400), (f) vimentin positivity in the tumor cells in pleural metastasis (×400)
References
- Wiemels J, Wrensch M, Claus EB. Epidemiology and etiology of meningioma. J Neurooncol 2010; 99: 307-14
- ed">2 Jääskeläinen J, Haltia M, Servo A. et al. Atypical and anaplastic meningiomas: Radiology, surgery, radiotherapy, and outcome. Surg Neurol 1986; 25: 233-42
- Surov A, Gottschling S, Bolz J, Kornhuber M, Alfieri A, Holzhausen HJ. et al. Distant metastases in meningioma: An underestimated problem. J Neurooncol 2013; 112: 323-7
- Karasick JL, Mullan SF. A survey of metastatic meningiomas. J Neurosurg 1974; 40: 206-12
- Louis DN, Perry A, Reifenberger G, von DeimlingA, Figarella-Branger D, Cavenee WK. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol 2016; 131: 803-20
- Kros JM, Cella F, Bakker SL, Paz Y, Geuze D, Egeler RM. Papillary meningioma with pleural metastasis: Case report and literature review. Acta Neurol Scand 2000; 102: 200-2
- Som PM, Sacher M, Strenger SW, Biller HF, Malis LI. “Benign” metastasizing meningiomas. AJNR Am J Neuroradiol 1987; 8: 127-30
- Kaminski JM, Movsas B, King E, Yang C, Kronz JD, Alli PM. et al. Metastatic meningioma to the lung with multiple pleural metastases. Am J Clin Oncol 2001; 24: 579-82
- Erman T, Hanta I, Haciyakupoǧlu S, Zorludemir S, Zeren H, Göçer AI. et al. Huge bilateral pulmonary and pleural metastasis from intracranial meningioma: A case report and review of the literature. J Neurooncol 2005; 74: 179-81
- Nakayama Y, Horio H, Horiguchi S, Hato T. Pulmonary and pleural metastases from benign meningeal meningioma: A case report. Ann Thorac Cardiovasc Surg 2014; 20: 410-3
- Yacoub M, Naccache JM, Kujas M, Valeyre D, Kambouchner M. Isolated pleural metastases from an atypical meningioma. Rev Mal Respir 2003; 20: 433-6


 PDF
PDF  Views
Views  Share
Share

