Report of chronic myeloid leukemia in chronic phase from Tata Memorial Hospital, Mumbai, 2002-2008
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2013; 34(03): 164-167
DOI: DOI: 10.4103/0971-5851.123716
Abstract
Background: Chronic myeloid leukemia (CML) is the commonest hematological malignancy in India. This manuscript is a single center analysis CML in chronic phase (CP). Materials and Methods: We did retrospective analysis of almost 1000 patients registered as chronic myeloid leukemia over a period of 6 years at Tata Memorial Hospital. Results: We found striking difference in cytogenetic response among patients presenting in late chronic phase (CP) compared with the patients in early CP. The rate of complete cytogenetic response among patients in late CP was 60% while in early CP it was 80%, which was statistically significant (P = 0.0001). The overall survival was 86%, at a median follow-up of 51 months. Innovator glivec was taken by 671 patients among which complete cytogenetic response (CCyR) was seen in 72% whereas generic veenat was taken by 237 patients and CCyR was seen in 75% of them. Conclusion: Availability of imatinib has dramatically changed the outlook for CML in India. The response was identical for those treated with innovator brand of imatinib as compared to the generic brand. Hence quality generics provide a cost effective solution, which is particularly relevant in the current global scenario.
Publication History
Article published online:
19 July 2021
© 2013. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:
Chronic myeloid leukemia (CML) is the commonest hematological malignancy in India. This manuscript is a single center analysis CML in chronic phase (CP).
Materials and Methods:
We did retrospective analysis of almost 1000 patients registered as chronic myeloid leukemia over a period of 6 years at Tata Memorial Hospital.
Results:
We found striking difference in cytogenetic response among patients presenting in late chronic phase (CP) compared with the patients in early CP. The rate of complete cytogenetic response among patients in late CP was 60% while in early CP it was 80%, which was statistically significant (P = 0.0001). The overall survival was 86%, at a median follow-up of 51 months. Innovator glivec was taken by 671 patients among which complete cytogenetic response (CCyR) was seen in 72% whereas generic veenat was taken by 237 patients and CCyR was seen in 75% of them.
Conclusion:
Availability of imatinib has dramatically changed the outlook for CML in India. The response was identical for those treated with innovator brand of imatinib as compared to the generic brand. Hence quality generics provide a cost effective solution, which is particularly relevant in the current global scenario.
INTRODUCTION
Oncology centers like Tata Memorial Hospital (TMH) see a large number of patients with CML every year. We have actually witnessed the revolutionary change in the treatment of CML from parenteral injections to simple oral medication that translated into dramatically improved survival as well as quality-of-life for these patients. With the assistance of patient assistance programs thousands of lives have been saved without the need for expensive, difficult and toxic treatment with interferon, cytarabine or Bone marrow transplantation. The introduction of imatinib (IM) and other oral tyrosine kinase inhibitors (TKIs) have improved understanding of the disease and led to change in the parameters of documenting disease response. [1,2] Complete blood counts are no longer accepted as response indicators by themselves; the value of cytogenetic and molecular response evaluation as well as mutation analysis is now well-established. [3,4] In this retrospective analysis, we document the outcome in one of the largest group of CML patients treated in India.
PATIENTS AND METHODS
The data was collected retrospectively from year January 2002 to January 2008. The files and the electronic medical record of all patients diagnosed with CML at the hospital provided information pertaining to age, sex, date of diagnosis, address, phone number, blood counts, bone marrow reports, spleen size at diagnosis, cytogenetic and molecular reports brand and dose of IM, change in dose, any primary or secondary resistance; tolerability, the side-effects of IM and outcome. Data was also obtained from records of patient assistance programs.
Definitions
Standard definitions for chronic phase (CP), accelerated phase (AP) and blast crisis were taken. For CP CML, primary resistance/treatment failure to IM (400 mg daily) was defined as failure to achieve any one of the following-complete hematologic response (CHR) after 3 months, cytogenetic response after 6 months, major cytogenetic response after 12 months and complete cytogenetic response (CCyR) after 18 months of therapy. Secondary resistance was defined as any one of the following-loss of CCyR or rising white blood cell count to >10 × 109/L on two occasions more than 4 weeks apart, progression to AP or progression to blast phases.
Statistical analysis
Event free survival (EFS) was defined as the time from initial diagnosis to primary/secondary resistance or death due to any cause. The statistical package for the social sciences system was used to analyses the data. The EFS and overall survival (OAS) probabilities were estimated using the Kaplan — Meier method and were compared using the log rank test. Differences among variables were evaluated using the χ2 test.
RESULTS
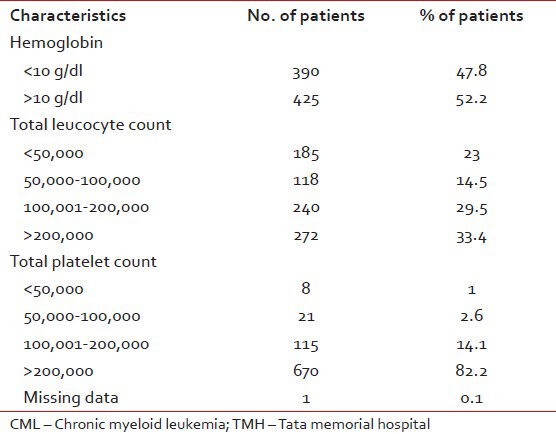
A total 972 patients of CML were registered between January 2002 and January 2008 with median age of 36 years (12-81 years). This included 730 (75%) males and 242 (25%) females, giving an M: F ratio of 3:1. Of these, 815 were evaluated at TMH at baseline. Their characteristics at diagnosis are shown in Table 1.
Table 1
The characteristics at diagnosis of CML patients evaluated at baseline at TMH (N = 815)
Response pattern
CHR
Documented CHR was available in 949 patients. Complete hematological response was documented in 937/949 (98.7%) patients while 12 (1.2%) patients showed primary resistance. Data was not available in 23 patients (2.4%).
CCyR
Documented cytogenetic response data was available in 908/972 (93%) patients. CCyR was seen in 699/908 (77%), absent in 209 (21.5%) and unknown in 64 (6.3%). [Table 2].
Table 2
CCyR with respect to various categories
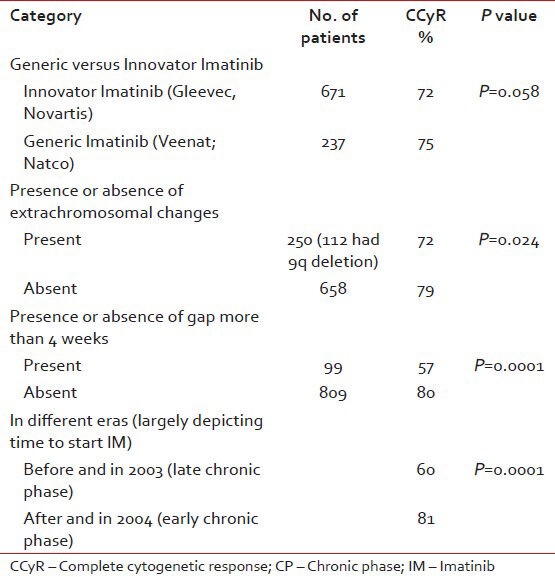
CCyR in generic versus Gleevec
CCyR was analyzed as per the brand of the IM used. It was seen that Glivec was taken by 671 patients and CCyR was seen in 72% while veenat was taken by 237 patients and CCyR was seen in 75% of patients.
CCyR rate in patients taken with gaps
It was found that irrespective of Brand, if there was more than 4 weeks gap of IM intake, the rate of CCyR was 57% and if it was less than 4 weeks the CCyR was 80%.
CCyR as per the era of treatment
The data was analyzed by dividing the patient population into two eras, i.e., pre- and post-2003. Majority of patients presenting before 2003 were exposed to hydroxyurea or cytarabine or interferon before getting IM, as it was introduced in TMH on regular basis in late 2003. This population was considered as patients in late CP and after 2003 as in early CP. And as expected the rate of CCyR before 2003 was 60% and after 2003 was 80%, which was statistically significant (P = 0.0001).
CCyR as per risk category and brand of IM
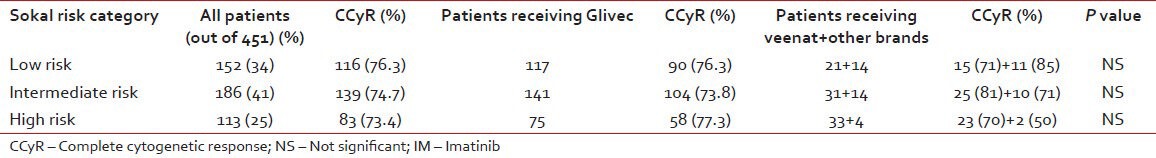
Table 3 shows the rate of response among various Sokal risk group categories. The CCyR was similar in all the three risk groups. The brand of IM used also did not seem to influence this outcome parameter.
Table 3
Comparison of response as per Sokal risk group and brand of IM
OAS
The OAS for whole population with median follow of 51 months was 86%.
At median follow-up of 51 months, the projected 5 year OAS for all patients was 86% [Figure 1]. The various events in population of 972 patients were as follows: In remission was 510 (53%), documented resistance or relapse was seen in 372 (38%), death occurred in 40 (4.1%), IM was stopped in 3 (0.3%) and lost to follow-up were 47 (4.8%) patients.
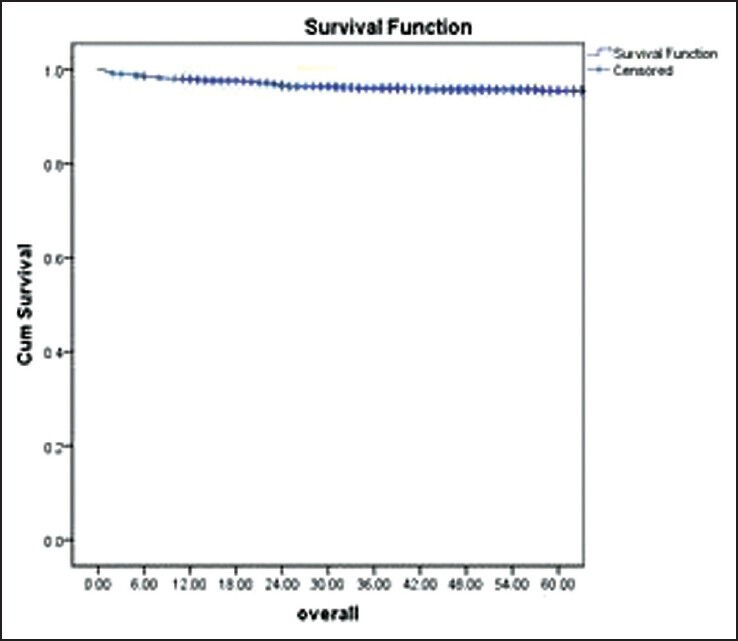
| Fig. 1 The overall survival of the patients diagnosed with chronic myeloid leukemia
DISCUSSION
Targeted therapy in the form of TKIs has completely changed the management and the outcome of CML. In this study, we made the comparisons between the groups like brand of IM, presence or absence of clonal evolution; time to start IM, continuity in taking IM. As this is retrospective data, it has its limitations. Nevertheless, it gives us few important points to ponder. Foremost, post-IM the OAS of our population is comparable with western population. We had OAS survival of 86% at 5 years, which was comparable with the landmark study, the international randomized study of interferon versus STI571 (IRIS) trial showing 89% OAS at 5 years [5] Second, our data showed that patients presenting in early CP do better when compared to patients in late CP. Again this finding is well documented in the literature. In a phase II trial by Gambacorti et al., they found patients in late CP had CCyR of 55% compared to 87% in early CP, as reported in IRIS trial. [6] Third, CCyR also depends on the continuation of IM intake. This data emphasizes that to have best results, it is important to minimize the gaps in IM intake and this can be achieved only by aggressive patient counseling and by optimizing the drug dosage on regular basis. The importance of IM adherence has been well-documented in ADAGIO study and data reconfirms it. [7] Fourth, the most important conclusion we can make in this study is that there is not much difference in the outcome of patients taking different brands of IM and generic can be used where original drug is not available to the patient.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES

| Fig. 1 The overall survival of the patients diagnosed with chronic myeloid leukemia


 PDF
PDF  Views
Views  Share
Share

