Study of Clinicopathological Spectrum and Pattern of Expression of Cyclooxygenase-2 in Urothelial Carcinomas of Bladder
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2018; 39(02): 172-177
DOI: DOI: 10.4103/ijmpo.ijmpo_81_17
Abstract
Introduction: Overexpression of cyclooxygenase-2 (COX2) in urothelial carcinoma of bladder (UCB) had been studied in the past by different workers and the results were contradictory. The objective of the present study was to evaluate the prognostic implication of COX2 expression in primary urothelial carcinomas of the urinary bladder and its correlation with clinical parameters, tumor stage, grade, and recurrence. Materials and methods: A total of 68 cases who underwent surgery for urothelial carcinoma in our medical college from January 2013 to December 2015 were evaluated in our study. Hematoxylin and eosin-stained slides were examined by two faculties applying standard reporting protocol. Tumor staging and grading was performed as per the WHO guidelines. Immunohistochemistry for expression of COX2 was performed to study any correlation of tumor grade with COX2 expression. The distribution of COX2 positivity was studied in tumors stratified according to established bladder cancer prognostic factor, for example, tumor size, grade, invasion, and spread. Results: Out of 68 cases, 42 cases showed COX2 positivity (61%). In low-grade cases of bladder carcinoma, COX2 positivity was 16 out of 24 cases (60%), and in high-grade cases, COX2 positivity was 10 out of 18 cases (64.28%).Conclusion: In the present study, the association of COX2 overexpression with advanced tumor invasion and tumor grade has been substantiated. Hence, COX2 expression can be taken as a prognostic factor along with other usual prognostic factors in patients of UCB.
Publication History
Article published online:
23 June 2021
© 2018. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Introduction: Overexpression of cyclooxygenase-2 (COX2) in urothelial carcinoma of bladder (UCB) had been studied in the past by different workers and the results were contradictory. The objective of the present study was to evaluate the prognostic implication of COX2 expression in primary urothelial carcinomas of the urinary bladder and its correlation with clinical parameters, tumor stage, grade, and recurrence. Materials and methods: A total of 68 cases who underwent surgery for urothelial carcinoma in our medical college from January 2013 to December 2015 were evaluated in our study. Hematoxylin and eosin-stained slides were examined by two faculties applying standard reporting protocol. Tumor staging and grading was performed as per the WHO guidelines. Immunohistochemistry for expression of COX2 was performed to study any correlation of tumor grade with COX2 expression. The distribution of COX2 positivity was studied in tumors stratified according to established bladder cancer prognostic factor, for example, tumor size, grade, invasion, and spread. Results: Out of 68 cases, 42 cases showed COX2 positivity (61%). In low-grade cases of bladder carcinoma, COX2 positivity was 16 out of 24 cases (60%), and in high-grade cases, COX2 positivity was 10 out of 18 cases (64.28%).Conclusion: In the present study, the association of COX2 overexpression with advanced tumor invasion and tumor grade has been substantiated. Hence, COX2 expression can be taken as a prognostic factor along with other usual prognostic factors in patients of UCB.
Introduction
Urothelial carcinoma of bladder (UCB) is the second most common genitourinary malignancy.[1],[2] It is the 7th most common malignancy in men and 17th in women.[3] The mortality of transitional cell carcinoma (TCC) of urinary bladder increases significantly with the progression of superficial or locally invasive disease (pTa/pT1) to detrusor muscle invasive disease (pT2).[4] Radical cystectomy with bilateral pelvic lymph node dissection is currently the gold standard treatment for muscle invasive UCB.[2],[5],[6] The most common prognostic markers in clinical use for these tumors are tumor stage and grade, which are subject to considerable intra- and inter-observer variation.[4] It is noted that about 40% of patients with organ-confined disease at the time of cystectomy subsequently suffer recurrence. Due to this high recurrence rate, there is a need for close follow-up throughout the patient's lifetime.[7]
Advanced pathological stage, nodal involvement, grade, and urinary obstruction have been reported as prognostic factors for survival and recurrence. However, many UCB with similar stage and grade have demonstrated variable clinical outcome after radical cystectomy.
Hence, many attempts have been made to determine new and reliable prognostic factors.[8],[9],[10],[11]
Many recent scientific studies show that chronic inflammation may positively influence the risk of UCB. Furthermore, studies investigating the prolonged use of cyclooxygenase-2 (COX2) inhibiting nonsteroidal anti-inflammatory drugs have reported a decrease in UCB risk.[12],[13]
Few studies have shown that bladder tissue from patients with cystitis or UCB exhibits elevated COX2 levels in contrast to benign bladder tissue.[14]
Furthermore, studies of tumor chemotherapy show that selective increase in tumor cytotoxicity relative to normal tissue can be achieved by inhibition of angiogenic inducers which are frequently present in bladder tumor. Several studies have correlated elevated vascular endothelial growth factor (VGEF) level or COX2 expression with disease recurrence or progression often as an independent prognostic factor by multivariate analysis.[15],[16]
This is the basis of combining anti-VGEF therapy or COX2 inhibitors with other forms of cytotoxic therapy in prospective clinical trials. While numerous study groups have investigated in different ways, the expressions of COX2 in UCB as a potential prognostic indicator, there is no clear consensus yet.[15],[16],[17],[18] Hence, our present study was formulated, and objective of our present study is to evaluate the prognostic implication of COX2 expression in primary urothelial carcinomas of the urinary bladder and its correlation with clinical parameters, tumor stage, grade, and recurrence.
Materials and Methods
A total of 68 cases who underwent surgery for TCC in our institute from January 2013 to December 2015 were included in the study.
Both transurethral resection and radical cystectomy cases were taken. Sections were examined by two faculties applying standard reporting protocol. Tumor staging and grading was performed as per the WHO guidelines.
Tumor differentiation, invasion depth, lymphovascular invasion (LVI), perineural invasion (PNI), necrosis, mitosis, and perivesical tumor spread were assessed histopathologically.
The association of age, gender, LVI, PNI, metastasis, necrosis, mitosis, and COX2 expression with pathological tumor grade and stage was evaluated and statistically analyzed.
Formalin-fixed, paraffin-embedded tissue sections were used for hematoxylin and eosin (H and E) staining and morphological diagnosis. A 2 μm thick paraffin-embedded section on poly-L-lysine-coated side was taken for immunohistochemistry (IHC). Antigen retrieval was done by heat treatment using microwave oven. IHC for COX2 expression was performed using rabbit monoclonal antibody to COX2 and Super Sensitive polymer-based detection system (Biogenex). Negative and positive controls were put up side by side.
Interpretation of COX2 immunostaining was then performed by two pathologists selecting the maximum positive area with strongest positive intensity and expressing it in a percentage.[19] The grading of COX2 expression was done semi-quantitatively. Positive staining pattern was graded as: undetected, mild (expressed in 10% tumor cells), moderate (10%–50% positive tumor cells), and strong (>50% positive tumor cells).[19],[20] For statistical analysis, undetected and mild cases were categorized as low and moderate and strong cases as high COX2 expression.[19] H and E-stained section was examined first and representative areas were identified. Those areas were examined in IHC slides by conventional microscopy.[19]
Results
Age range of the patients included in the study was 45–82 years with median 60.20 years. Out of 68 patients, 56 were male and 12 female. Five patients were below 50 years of age (all male). Hence, our data are confirming the fact that bladder carcinoma is more common in male compared to female patients [5] [Table 1].
|
Age |
Total number |
COX2-positive cases |
COX2-negative cases |
|---|---|---|---|
|
COX2 – Cyclooxygenase-2 |
|||
|
<50> |
5 (all males) |
4 (male - 4) |
1 (male - 1) |
|
50 years |
63 (female - |
38 (female - |
25 (female - 5, |
|
or more |
12, male - 51) |
7, male - 31) |
male - 20) |
|
Tumor cases |
Total number |
COX2-positive cases |
COX2-negative cases |
|---|---|---|---|
|
COX2 – Cyclooxygenase-2 |
|||
|
3 cm or less |
26 |
14 (53.8) |
12 |
|
>3 cm |
30 |
20 (66.6) |
10 |
|
Tumor grade |
Total cases |
COX2-positive cases |
COX2-negative cases |
|---|---|---|---|
|
COX2 – Cyclooxygenase-2 |
|||
|
low grade |
40 |
24 (60) |
16 |
|
High grade |
28 |
18 (64.28) |
10 |
|
Tumor invasion |
Total cases |
COX2-positive cases |
COX2-negative cases |
|---|---|---|---|
|
COX2 – Cyclooxygenase-2 |
|||
|
T2 |
25 |
12 (48) |
13 |
|
T3 |
24 |
16 (66.6) |
08 |
|
T4 |
19 |
14 (73.6) |
05 |
|
Lymphatic invasion |
Total cases |
COX2-positive cases |
COX2-negative cases |
|---|---|---|---|
|
COX2 – Cyclooxygenase-2 |
|||
|
N0 |
18 |
11 (61.11) |
7 |
|
N1, N3 |
36 |
24 (66.6) |
12 |
|
Nx |
14 |
5 |
9 |
|
Lymphatic invasion |
Total cases |
COX2-positive cases |
COX2-negative cases |
|---|---|---|---|
|
COX2 – Cyclooxygenase-2 |
|||
|
N0 |
18 |
11 (61.11) |
7 |
|
N1, N3 |
36 |
24 (66.6) |
12 |
|
Nx |
14 |
5 |
9 |
|
Metastasis |
Total cases |
COX2-positive cases |
COX2-negative cases |
|---|---|---|---|
|
COX2 – Cyclooxygenase-2 |
|||
|
M0 |
20 |
12 (60) |
8 |
|
M1 |
20 |
15(75) |
15 |
|
Mx |
28 |
15 (53.5) |
13 |
|
COX2 expression |
Tumor necrosis (%) |
Concomitant carcinoma in situ (%) |
|---|---|---|
|
COX2 – Cyclooxygenase-2 |
||
|
Negative |
36 (52.9) |
40 (58.8) |
|
Positive |
32 (47) |
28 (41.1) |
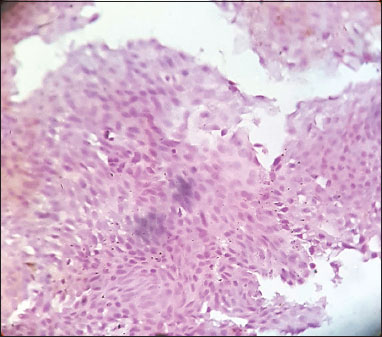
| Figure.1Low-grade urothelial carcinoma, immunohistochemistry showing negative cyclooxygenase-2 [removed]×100)
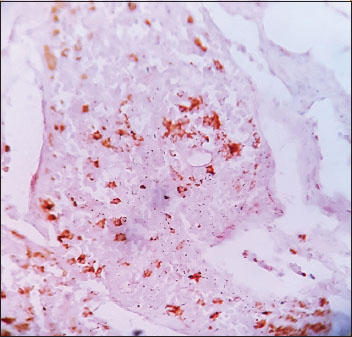
| Figure.2Low-grade urothelial carcinoma, immunohistochemistry showing weak cyclooxygenase-2 [removed]×100)
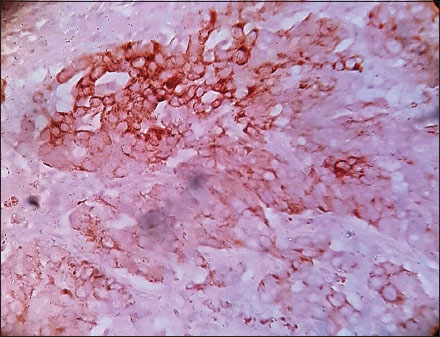
| Figure.3High-grade urothelial carcinoma, immunohistochemistry showing strong cyclooxygenase-2 [removed]×400)
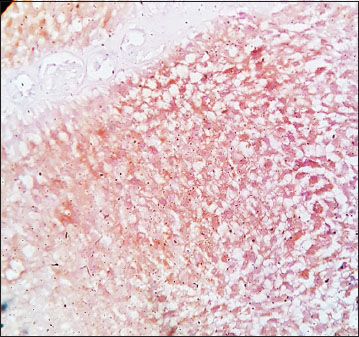
| Figure.4High-grade urothelial carcinoma, immunohistochemistry showing moderate cyclooxygenase-2 [removed]×400)
|
LVI |
Total cases |
COX2-positive cases |
COX2-negative cases |
|---|---|---|---|
|
LVI – Lymphovascular invasion; COX2 – Cyclooxygenase 2 |
|||
|
Present |
40 32 (80) |
8 |
|
|
Absent |
28 10 (35.7) |
18 |
|
|
PNI |
Total cases |
COX2-positive cases |
COX2-negative cases |
|---|---|---|---|
|
LVI – Lymphovascular invasion; COX2 – Cyclooxygenase 2 |
|||
|
Present |
35 |
26 (74.28) |
9 |
|
Absent |
33 |
16 (48.4) |
17 |

| Figure.1Low-grade urothelial carcinoma, immunohistochemistry showing negative cyclooxygenase-2 [removed]×100)

| Figure.2Low-grade urothelial carcinoma, immunohistochemistry showing weak cyclooxygenase-2 [removed]×100)

| Figure.3High-grade urothelial carcinoma, immunohistochemistry showing strong cyclooxygenase-2 [removed]×400)

| Figure.4High-grade urothelial carcinoma, immunohistochemistry showing moderate cyclooxygenase-2 [removed]×400)
References
- May M, Bastian PJ, Brookman-May S, Fritsche HM, Tilki D, Otto W. et al. Gender-specific differences in cancer-specific survival after radical cystectomy for patients with urothelial carcinoma of the urinary bladder in pathologic tumor stage T4a. Urol Oncol 2013; 31: 1141-7
- Bruins HM, Arends TJ, Pelkman M, Hulsbergen-van de KaaCA, van der HeijdenAG, Witjes JA. et al. Radical cystectomy in a Dutch University hospital: Long-term outcomes and prognostic factors in a homogeneous surgery-only series. Clin Genitourin Cancer 2014; 12: 190-5
- Ploeg M, Aben KK, Kiemeney LA. The present and future burden of urinary bladder cancer in the world. World J Urol 2009; 27: 289-93
- Latif Z, Watters AD, Dunn I, Grigor KM, Underwood MA, Bartlett JM. HER2/neu overexpression in the development of muscle-invasive transitional cell carcinoma of the bladder. Br J Cancer 2003; 89: 1305-9
- Otto W, May M, Fritsche HM, Dragun D, Aziz A, Gierth M. et al. Analysis of sex differences in cancer-specific survival and perioperative mortality following radical cystectomy: Results of a large German multicenter study of nearly 2500 patients with urothelial carcinoma of the bladder. Gend Med 2012; 9: 481-9
- May M, Stief C, Brookman-May S, Otto W, Gilfrich C, Roigas J. et al. Gender-dependent cancer-specific survival following radical cystectomy. World J Urol 2012; 30: 707-13
- Botteman MF, Pashos CL, Redaelli A, Laskin B, Hauser R. The health economics of bladder cancer: A comprehensive review of the published literature. Pharmacoeconomics 2003; 21: 1315-30
- Hong SK, Kwak S, Jeon HG, Lee E, Lee SE. Do vascular, lymphatic, and perineural invasion have prognostic implications for bladder cancer after radical cystectomy?. Urology 2005; 65: 697-702
- Zigeuner R, Shariat SF, Margulis V, Karakiewicz PI, Roscigno M, Weizer A. et al. Tumour necrosis is an indicator of aggressive biology in patients with urothelial carcinoma of the upper urinary tract. Eur Urol 2010; 57: 575-81
- Xylinas E, Rink M, Robinson BD, Lotan Y, Babjuk M, Brisuda A. et al. Impact of histological variants on oncological outcomes of patients with urothelial carcinoma of the bladder treated with radical cystectomy. Eur J Cancer 2013; 49: 1889-97
- Mitra AP, Bartsch CC, Bartsch GJr, Miranda G, Skinner EC, Daneshmand S. Does presence of squamous and glandular differentiation in urothelial carcinoma of the bladder at cystectomy portend poor prognosis? An intensive case-control analysis. Urol Oncol 2014; 32: 117-27
- Wheeler MA, Hausladen DA, Yoon JH, Weiss RM. Prostaglandin E2 production and cyclooxygenase-2 induction in human urinary tract infections and bladder cancer. J Urol 2002; 168(4 Pt 1): 1568-73
- Shirahama T. Cyclooxygenase-2 expression is up-regulated in transitional cell carcinoma and its preneoplastic lesions in the human urinary bladder. Clin Cancer Res 2000; 6: 2424-30
- Diamantopoulou K, Lazaris A, Mylona E, Zervas A, Stravodimos K, Nikolaou I. et al. Cyclooxygenase-2 protein expression in relation to apoptotic potential and its prognostic significance in bladder urothelial carcinoma. Anticancer Res 2005; 25: 4543-9
- Eltze E, Wülfing C, Von StruenseeD, Piechota H, Buerger H, Hertle H. Cox-2 and Her2/neu co-expression in invasive bladder cancer. Int J Oncol 2005; 26: 1525-31
- Friedrich MG, Toma MI, Petri S, Huland H. Cyclooxygenase-2 promotes angiogenesis in pTa/T1 urothelial bladder carcinoma but does not predict recurrence. BJU Int 2003; 92: 389-92
- Gudjónsson S, Bendahl PO, Chebil G, Höglund M, Lindgren D, Lundberg LM. et al. Can tissue microarray-based analysis of protein expression predict recurrence of stage Ta bladder cancer?. Scand J Urol Nephrol 2011; 45: 270-7
- Hilmy M, Campbell R, Bartlett JM, McNicol AM, Underwood MA, McMillan DC. The relationship between the systemic inflammatory response, tumour proliferative activity, T-lymphocytic infiltration and COX-2 expression and survival in patients with transitional cell carcinoma of the urinary bladder. Br J Cancer 2006; 95: 1234-8
- Czachorowski MJ, Amaral AF, Montes-Moreno S, Lloreta J, Carrato A, Tardón A. et al. Cyclooxygenase-2 expression in bladder cancer and patient prognosis: Results from a large clinical cohort and meta-analysis. PLoS One 2012; 7: e45025
- Hsu FS, Hsin C, Tu PY, Pu SY. The role of cyclooxygenese-2 expression in chemoresistance and invasiveness of urothelial carcinoma. Imp J Interdiscip Res 2016; 2: 979-86
- Fajkovic H, Halpern JA, Cha EK, Bahadori A, Chromecki TF, Karakiewicz PI. et al. Impact of gender on bladder cancer incidence, staging, and prognosis. World J Urol 2011; 29: 457-63
- Cao D, Vollmer RT, Luly J, Jain S, Roytman TM, Ferris CW. et al. Comparison of 2004 and 1973 World Health Organization grading systems and their relationship to pathologic staging for predicting long-term prognosis in patients with urothelial carcinoma. Urology 2010; 76: 593-9
- Babjuk M, Oosterlinck W, Sylvester R, Kaasinen E, Böhle A, Palou-Redorta J. et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder, the 2011 update. Eur Urol 2011; 59: 997-1008
- Stein JP, Quek ML, Skinner DG. Lymphadenectomy for invasive bladder cancer: I. historical perspective and contemporary rationale. BJU Int 2006; 97: 227-31
- Lotan Y, Gupta A, Shariat SF, Palapattu GS, Vazina A, Karakiewicz PI. et al. Lymphovascular invasion is independently associated with overall survival, cause-specific survival, and local and distant recurrence in patients with negative lymph nodes at radical cystectomy. J Clin Oncol 2005; 23: 6533-9
- Hara S, Miyake H, Fujisawa M, Okada H, Arakawa S, Kamidono S. et al. Prognostic variables in patients who have undergone radical cystectomy for transitional cell carcinoma of the bladder. Jpn J Clin Oncol 2001; 31: 399-402
- Leissner J, Koeppen C, Wolf HK. Prognostic significance of vascular and perineural invasion in urothelial bladder cancer treated with radical cystectomy. J Urol 2003; 169: 955-60
- Yoshimura R, Sano H, Mitsuhashi M, Kohno M, Chargui J, Wada S. Expression of cyclooxygenase-2 in patients with bladder carcinoma. J Urol 2001; 165: 1468-72
- Wild PJ, Kunz-Schughart LA, Stoehr R, Burger M, Blaszyk H, Simon R. et al. High-throughput tissue microarray analysis of COX2 expression in urinary bladder cancer. Int J Oncol 2005; 27: 385-91
- Wülfing C, Eltze E, von Struensee D, Wülfing P, Hertle L, Piechota H. Cyclooxygenase-2 expression in bladder cancer: Correlation with poor outcome after chemotherapy. Eur Urol 2004; 45: 46-52
- Margulis V, Shariat SF, Ashfaq R, Thompson M, Sagalowsky AI, Hsieh JT. Expression of cyclooxygenase-2 in normal urothelium, and superficial and advanced transitional cell carcinoma of bladder. J Urol 2007; 177: 1163-8
- Shirahama T, Arima J, Akiba S, Sakakura C. Relation between cyclooxygenase-2 expression and tumor invasiveness and patient survival in transitional cell carcinoma of the urinary bladder. Cancer 2001; 92: 188-93
- Ferrandina G, Lauriola L, Zannoni GF, Distefano MG, Legge F, Salutari V. et al. Expression of cyclooxygenase-2 (COX-2) in tumour and stroma compartments in cervical cancer: Clinical implications. Br J Cancer 2002; 87: 1145-52
- Matsuo T, Miyata Y, Mipsunari K. Pathological significance and prognostic implications of heme oxygenese1 expression in non muscle invasive bladder cancer: Correlation with cell proliferation, angiogenesis, lymphangiogenesis and expression of VEGFs and cox2. Oncol Lett 2017; 13: 275-80
- Prabhu B, Balakrishnan D, Sundaresan S. Antiproliferative and anti-inflammatory properties of diindolylmethane and lupeol against N-butyl-N-(4-hydroxybutyl) nitrosamine induced bladder carcinogenesis in experimental rats. Hum Exp Toxicol 2016; 35: 685-92


 PDF
PDF  Views
Views  Share
Share

