Toxoplasmosis and Lymphoma: The Mimickers
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(02): 248-250
DOI: DOI: 10.4103/ijmpo.ijmpo_155_16

|
Publication History
Article published online:
06 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Sir,
Cervical lymphadenopathy (CL) is a common clinical finding with a broad differential diagnosis and is frequently benign. Benign etiologies of CL which can mimic lymphomas include: infections like HIV or Epstein–Barr virus (EBV); autoimmune, hypersensitivity, or other benign disorders including sarcoidosis; benign reactive lymphadenopathies, like Kikuchi's disease; and atypical potentially malignant lymphoproliferative disorders, including Castleman's disease and lymphomatoid granulomatosis. We describe a 45-year-old woman who had coexisting toxoplasmosis and diffuse large B-cell lymphoma (DLBCL) causing diagnostic dilemma.
A 45-year-old previously healthy housewife was admitted with high-grade intermittent fever and bilateral neck swelling for 1 month. She used to live with cats. She had a history of significant weight loss, had no sick contacts and had no history of addictions. On examination, she had a fever of 100°F, tachycardia, and normal blood pressure. She had mild pallor and multiple lymph nodes in submandibular and supraclavicular regions bilaterally; largest measuring 2 cm × 2 cm, firm, nontender, and matted. Hemoglobin was 11.3 g/dl, total leukocyte count 8400/ml with normal differential, platelet count 230,000/μL, erythrocyte sedimentation rate 47 mm in 1 h. Blood chemistries were normal. Chest X-ray was normal. Purified protein derivative test was negative. HIV, hepatitis B and hepatitis C serologies were negative. Ultrasonography (USG) of the neck showed multiple enlarged cervical lymph nodes largest of 3.8 cm × 1.9 cm in the left submandibular region, hypoechoic with central cystic areas. Power Doppler sonography was normal. Cervical lymph node biopsy showed numerous microgranulomas [Figure 1]. Toxoplasma IgG and IgM antibody titers were high. A 5-fold increase in IgG antibody titer was seen in serial sample obtained 3 weeks later. She was diagnosed to have CL secondary to toxoplasmosis and was started on cotrimoxazole.
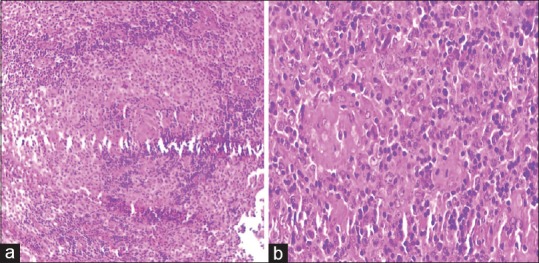
| Figure 1:Cervical lymph node biopsy showing microgranuloma (a) H and E, ×10, (b) H and E, ×40
One month after starting cotrimoxazole, she was readmitted with continuing fever and increase in size of the cervical nodes. Her blood counts and chemistries were normal. Peripheral smear was normal. A repeat lymph node biopsy showed effacement of architecture replaced diffusely by large atypical cells (CD20 positive and CD3, CD15, CD30, CD10, S100, Bcl 6, and MIB negative) with eosinophilic cytoplasm, ill-defined cell borders, irregular/round vesicular nuclei and eosinophilic nucleoli; suggesting DLBCL [Figures [Figures22 and and3].3]. Contrast-enhanced computed tomography of chest and abdomen, and bone marrow study were normal. She was treated with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone.
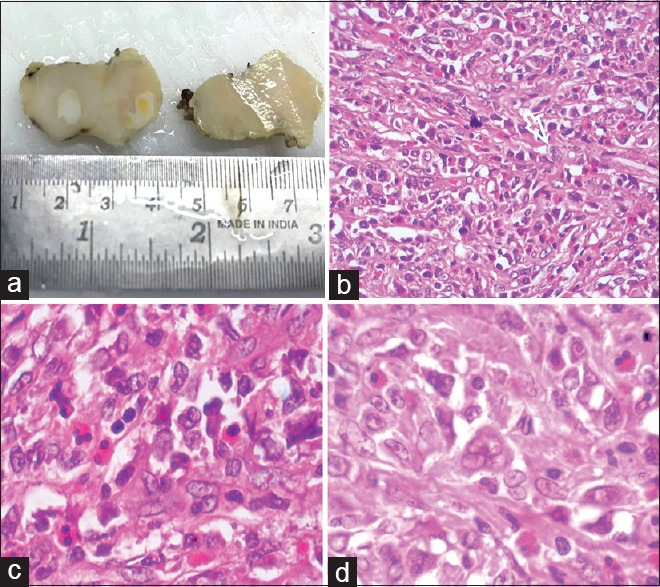
| Figure 2:Cervical lymph node biopsy showing effacement of architecture, replaced diffusely by large atypical cells with eosinophilic cytoplasm, ill-defined cell borders, irregular/round vesicular nuclei and eosinophilic nucleoli; suggesting diffuse large B-cell lymphoma (a) cut section, (b) H and E, ×40, (c and d) H and E, ×100
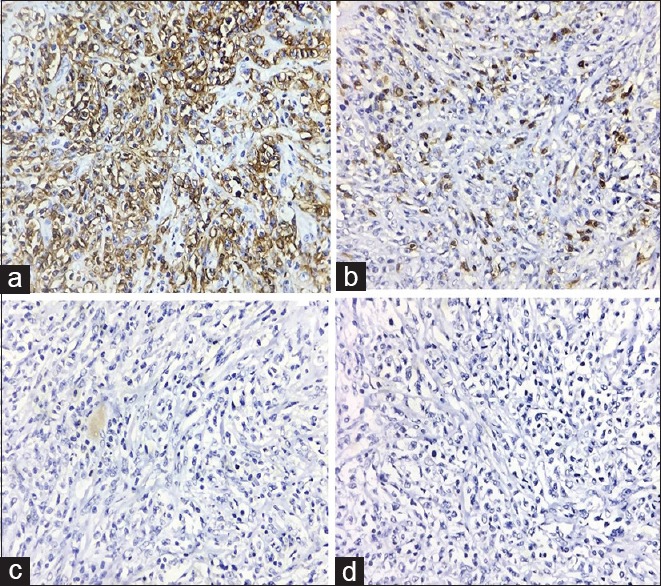
| Figure 3:The abnormal cells showing (a) CD20 positivity and (b-d) CD3, CD15, CD30 negativity
Infection with Toxoplasma gondii, an obligate intracellular protozoan is usually asymptomatic in immunocompetent. T. gondii has a complex life cycle and cats are the definitive host, where the sexual stage of reproduction occurs. Intermediate hosts for the organism, including mammals and humans, are then infected, usually through ingestion of oocysts contained in contaminated soil or infected raw or undercooked meat. Nearly 10%–20% of immunocompetent patients can have, a nonspecific and self-limiting illness manifested most typically by isolated CL or occipital lymphadenopathy lasting for <4 href="https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5582575/#ref1" rid="ref1" class=" bibr popnode tag_hotlink tag_tooltip" id="__tag_635940920" role="button" aria-expanded="false" aria-haspopup="true" xss=removed>1,2] The patient had exposure to cats and, had histopathological and serological evidence for toxoplasma infection.
Benign causes of lymphadenopathy can include infections, autoimmune disorders, drug hypersensitivity reactions, sarcoidosis, and amyloidosis.[3] Infections commonly associated with persistent, multifocal lymphadenopathy are toxoplasmosis, tuberculosis, EBV, and HIV.[4] Immunity to toxoplasma depends principally on T-lymphocytes and to a much lesser degree on antibody production.[5] In patients with hematological malignancies, stem cell or bone marrow transplant and T- and B-cell depleting regimens are probably the important risk factor for reactivation of a latent toxoplasma infection. Impaired immunity in lymphoma patients can predispose them for infections like toxoplasmosis which may arise de novo or secondary to reactivation of latent infection.
USG of lymphnodes may help in identifying malignant lymph nodes in the neck including metastases and lymphoma. Gray scale sonographic features that help to identify metastatic and lymphomatous lymph nodes include size, shape and internal architecture (loss of hilar architecture, presence of intranodal necrosis, and calcification). Doppler sonography of lymphomatous lymph nodes tends to have both hilar and peripheral vessels. The high incidence of hilar vascularity in lymphomatous nodes is thought to be related to the fact that intranodal necrosis or keratinization is not common in lymphoma, and therefore, the hilar vessels of the nodes are preserved.[6] Advances in ultrasound (US) technology, such as contrast-enhanced US, contrast-enhanced endoscopic US, and real-time elastography show potential to improve the accuracy of US for the differential diagnosis of benign and malignant lymph nodes.[7] The patient did not have any ultrasonographic features suggesting lymphoma.
Coexistence of two different etiologies for CL can cause a diagnostic dilemma, as in our case there was no evidence to suspect the presence of an underlying malignancy when the patient was evaluated for the first time. Only when the patient had an increase in the size of the lymphnodes an alternative cause for CL was considered. A repeat lymph node biopsy revealed lymphoma. CL have diverse causes, both benign and malignant which may coexist very rarely masking one another. We describe a 45-year-old woman who had both benign and malignant causes for CL together to help the readers to be aware of such rare presentations.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Robert-Gangneux F, Dardé ML. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev 2012;25:264-96.
- Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet 2004;363:1965-76.
- Brown JR, Skarin AT. Clinical mimics of lymphoma. Oncologist 2004;9:406-16.
- Chau I, Kelleher MT, Cunningham D, Norman AR, Wotherspoon A, Trott P, et al. Rapid access multidisciplinary lymph node diagnostic clinic: Analysis of 550 patients. Br J Cancer 2003;88:354-61.
- Dupont CD, Christian DA, Hunter CA. Immune response and immunopathology during toxoplasmosis. Semin Immunopathol 2012;34:793-813.
- Ahuja AT, Ying M, Ho SY, Antonio G, Lee YP, King AD, et al. Ultrasound of malignant cervical lymph nodes. Cancer Imaging 2008;8:48-56.
- Cui XW, Jenssen C, Saftoiu A, Ignee A, Dietrich CF. New ultrasound techniques for lymph node evaluation. World J Gastroenterol 2013;19:4850-60.

| Figure 1:Cervical lymph node biopsy showing microgranuloma (a) H and E, ×10, (b) H and E, ×40

| Figure 2:Cervical lymph node biopsy showing effacement of architecture, replaced diffusely by large atypical cells with eosinophilic cytoplasm, ill-defined cell borders, irregular/round vesicular nuclei and eosinophilic nucleoli; suggesting diffuse large B-cell lymphoma (a) cut section, (b) H and E, ×40, (c and d) H and E, ×100

| Figure 3:The abnormal cells showing (a) CD20 positivity and (b-d) CD3, CD15, CD30 negativity
References
- Robert-Gangneux F, Dardé ML. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev 2012;25:264-96.
- Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet 2004;363:1965-76.
- Brown JR, Skarin AT. Clinical mimics of lymphoma. Oncologist 2004;9:406-16.
- Chau I, Kelleher MT, Cunningham D, Norman AR, Wotherspoon A, Trott P, et al. Rapid access multidisciplinary lymph node diagnostic clinic: Analysis of 550 patients. Br J Cancer 2003;88:354-61.
- Dupont CD, Christian DA, Hunter CA. Immune response and immunopathology during toxoplasmosis. Semin Immunopathol 2012;34:793-813.
- Ahuja AT, Ying M, Ho SY, Antonio G, Lee YP, King AD, et al. Ultrasound of malignant cervical lymph nodes. Cancer Imaging 2008;8:48-56.
- Cui XW, Jenssen C, Saftoiu A, Ignee A, Dietrich CF. New ultrasound techniques for lymph node evaluation. World J Gastroenterol 2013;19:4850-60.


 PDF
PDF  Views
Views  Share
Share

