Transforming growth factor-β1 and TGF-β2 act synergistically in the fibrotic pathway in oral submucous fibrosis: An immunohistochemical observation
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2015; 36(02): 111-116
DOI: DOI: 10.4103/0971-5851.158842
Abstract
Background and objectives: Oral Submucous Fibrosis (OSF) is a potentially malignant oral disorder which leads to fibrosis of the oral mucosa and has a high rate of malignant transformation. The consumption of various forms of areca nut is causatively linked to the condition. The constituents of areca nut activate several pro-fibrotic cytokines, chiefly transforming growth factor-β1, β2, which leads to an increased deposition and decreased degradation of extracellular matrix and collagen. TGF-β1, β2 probably represent the major pathway in the deposition of collagen fibres in this condition. The present study aims to identify and correlate the expressions of TGF-β1 and TGF-β2 immunohistochemically on paraffin sections of various stages of OSF. A comparison was also made between normal oral mucosa and scar tissue and OSF to judge the mode, extent and type of expression of TGF β1, β2. Methods: The expression of TGF-β1 antibody (8A11, NovusBio, USA) and TGF-β2 antibody (TB21, NovusBio, USA) was detected immunohistochemically on paraffin sections of 58 and 70 cases of OSF respectively, 10 cases of normal oral mucosal tissue and 4 cases of scar tissue. A mapping of the positivity of the two cytokines was done using JenOptik camera and ProReg image analysis software. The results were statistically analysed using one way ANOVA and students "t" test. Results: Expression of TGF-β1 and TGF-β2 was more in OSF as compared with normal oral mucosa, scar/keloid tissue showing highest values. Positivity for both the markers was seen in epithelium, around the blood vessels, in areas of inflammatory infiltrate, fibroblasts and in muscles. TGF-β1 expression was higher and more intense than that of TGF-β2 in all the cases. TGF-β2 was restricted in its expression to submucosal area with minimal involvement of the epithelium and the deeper muscle tissue. Conclusion: TGF-β1 is the most prominent cytokine in the fibrotic pathway and TGF-β2 plays a contributory role.
Keywords
Immunohistochemistry - oral submucous fibrosis - scar - transforming growth factor-beta1 - transforming growth factor-beta2Publication History
Article published online:
12 July 2021
© 2015. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background and objectives:
Oral Submucous Fibrosis (OSF) is a potentially malignant oral disorder which leads to fibrosis of the oral mucosa and has a high rate of malignant transformation. The consumption of various forms of areca nut is causatively linked to the condition. The constituents of areca nut activate several pro-fibrotic cytokines, chiefly transforming growth factor-β1, β2, which leads to an increased deposition and decreased degradation of extracellular matrix and collagen. TGF-β1, β2 probably represent the major pathway in the deposition of collagen fibres in this condition. The present study aims to identify and correlate the expressions of TGF-β1 and TGF-β2 immunohistochemically on paraffin sections of various stages of OSF. A comparison was also made between normal oral mucosa and scar tissue and OSF to judge the mode, extent and type of expression of TGF β1, β2.
Methods:
The expression of TGF-β1 antibody (8A11, NovusBio, USA) and TGF-β2 antibody (TB21, NovusBio, USA) was detected immunohistochemically on paraffin sections of 58 and 70 cases of OSF respectively, 10 cases of normal oral mucosal tissue and 4 cases of scar tissue. A mapping of the positivity of the two cytokines was done using JenOptik camera and ProReg image analysis software. The results were statistically analysed using one way ANOVA and students “t” test.
Results:
Expression of TGF-β1 and TGF-β2 was more in OSF as compared with normal oral mucosa, scar/keloid tissue showing highest values. Positivity for both the markers was seen in epithelium, around the blood vessels, in areas of inflammatory infiltrate, fibroblasts and in muscles. TGF-β1 expression was higher and more intense than that of TGF-β2 in all the cases. TGF-β2 was restricted in its expression to submucosal area with minimal involvement of the epithelium and the deeper muscle tissue.
Conclusion:
TGF-β1 is the most prominent cytokine in the fibrotic pathway and TGF-β2 plays a contributory role.
INTRODUCTION
Oral submucous fibrosis (OSF) is a collagen-related disorder induced by betel quid chewing habit. Fibro elastic changes are seen in sub epithelial layer due to abnormal accumulation of collagen resulting in dense fibrotic bands in the mouth. A number of factors trigger the disease process by causing a juxtaepithelial inflammatory reaction in the oral mucosa.[1]
The pathogenesis of the disease is not well established and is believed to be multifactorial. Contents of arecanut especially alkaloids like arecaidine and arecoline are believed to trigger the deposition of collagen. The pathognomic fibrosis in the disorder follows a standard postinflammatory reaction course but probably deviates resulting in overproduction and decreased degradation.
Synthesis of collagen is influenced by a variety of mediators, including growth factors, hormones, cytokines and lymphokines.[2] Transforming growth factor-beta (TGF-β) is a pro-fibrotic growth factor implicated in the development of fibrotic lesions. It causes deposition of extracellular matrix (ECM) by increasing the synthesis of matrix protein like collagen and decreasing the degradation by stimulating various inhibitor mechanisms. TGF-β represents large family of growth and differentiation factors that mobilize through complex signaling networks to regulate cellular differentiation, proliferation, motility, adhesion and apoptosis. Although TGF-β is essential for healing, overproduction leads to scar tissue and fibrosis. TGF-β1 isoform is most implicated in fibrosis.[3] This peptide plays a critical role not only in synthesis and degradation of ECM but also in response of cells to ECM mediated through integrin receptors; moreover, specific components of the ECM, in turn, can both deliver TGF-β and regulate its activity. Isoforms of TGF-β, β1 and β2 have been linked to variations of protein expression or function. TGF-β1 is a key regulator of ECM assembly and remodeling. The cytokine TGF-β1 is considered to have a central role in inducing myofibroblastic phenotype, and its expression is increased under numerous fibrotic conditions. Thus TGF-β signaling pathway might be critical for pathogenesis of OSF. That TGF-β stimulates fibroblast proliferation and EM elaboration suggests the importance of these cytokines in fibroelastic diseases.[1]
The present study aimed to identify and correlate the expressions of TGF-β1 and TGF-β2 immunohistochemically in various stages of OSF in paraffin embedded sections. The expression of the cytokines was also identified in normal oral mucosa and scar tissue for a comparative analyses.
MATERIALS AND METHODS
The present study involved archival material of OSF, normal buccal mucosa and scar tissue. Paraffin sections of OSF were stained with TGF-β1- (n = 58), TGF-β2-antibody (n = 70). OSF cases were histologically graded according to Pindborg and Sirsat[4] classification. 10 cases of normal buccal mucosa were assessed for both the isoforms. A comparative analyses was done using scar tissue; TGF-β1- (n = 5) and TGF-β2-antibody (n = 3).
Hematoxylin and eosin stained sections of 4 μ thickness were made for routine histological examination. For immunohistochemical examination, 4 μ sections were used and the staining procedure followed was based on instructions provided by the manufacturer.
Mouse monoclonal antibodies, anti-TGF-β1 (8A11) (NovusBio®, USA) (Cat No: NB110-59988) and anti-TGF-β2 (TB21) (NovusBio®, USA) (Cat No: NBP1-51749) were used for the study. The immunohistochemical procedure utilized a super sensitive polymer kit (BioGenex Life Sciences Ltd®, CA, USA) that uses a nonbiotin polymeric technology wherein the secondary antibody conjugated to poly-HRP (horse-radish peroxidase) reagent is bound to the primary antibody and then visualized by the chromogen. Background intensity was enhanced using antibody diluent with background reducing components (Dako®, Denmark). The dilution was followed according to the manufacturer's specification that is, 4 μl/100 μl for anti-TGF-β1 and 1 μl/100 μl for anti-TGF-β2 antibody. The diluted antibody was stored in the refrigerator at 2-4°C if necessary or it was diluted as and when required.
Four micron thick paraffin sections were mounted on silane-coated slides and stained with anti-TGF-β1 and anti-TGF-β2 antibody as per the manufacturer's directions. Antigen retrieval was done in a standard microwave using Tris-buffer. Scar tissue was used a positive control and omission of the primary antibody was used as the negative control. The site of localization of the antibody was noted and tabulated.
Microscopic images were captured using a JenOptix 500 (Lawrence and Mayo Inc. USA) camera attached to the microscope and were analysed using ProReg® Capture Pro 2.8.8 software (2011, Jenoptik AG, USA). The area of positivity was charted out and statistically compared within parameters of total tissue area and various sites in the tissue. The parameters charted out included:
- P_SM — Proportional expression in the submucosa.
- P_M — Proportional expression in the muscle.
- SM_T — Expression in submucosa in relation to expression in the whole of the tissue.
- M_T — Expression in muscle in relation to expression in the whole of the tissue.
- SM_M — Expression in submucosa in relation to that in muscle area.
The computation in the muscle could be done only in biopsies of adequate depth where muscle was present.
RESULTS
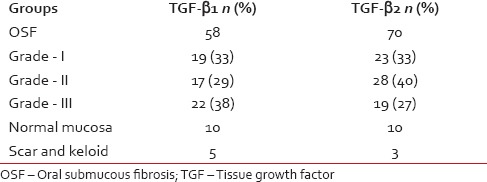
Table 1 gives the grade-wise distribution of the samples included in the study.
Table 1
Grade wise distribution of OSF cases, control and scar tissue
Epithelium
The expression of the biomarkers was quantified with image analysis software. Based on the results a proportional site-wise quantification was done. Table 2 depicts the results of the plotting. Interestingly in the epithelium, in all the groups, expression of TGF-β1 was highest in the basal-suprabasal layers followed by the spinous layer. TGF-β2 was expressed more in terms of area as compared with TGF-β1 and was restricted to the basal-parabasal and the spinous layers [Figures [Figures11–4].
Table 2
Mapping of positive expression of TGF-β1 and (TGF-β2)@ of the IHC stained tissue as computed by image analysis#
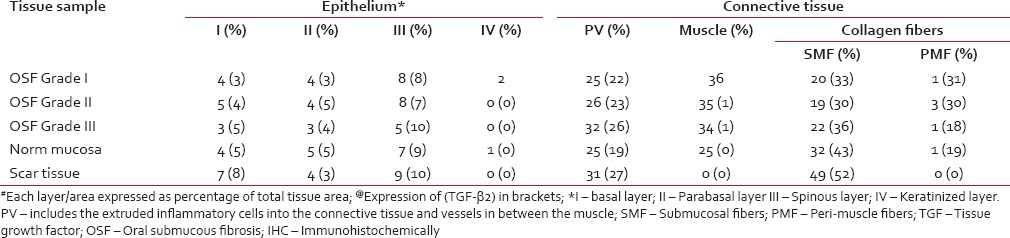
| Fig. 1 Grade I oral submucous fibrosis tissue stained with antitransforming growth factor-beta1 (TGF-β1) (left side picture) and anti-TGF-β2 (right side picture). Note intensity and extent of TGF-β1 expression especially in the deeper layers and epithelium (original magnification, ×10)
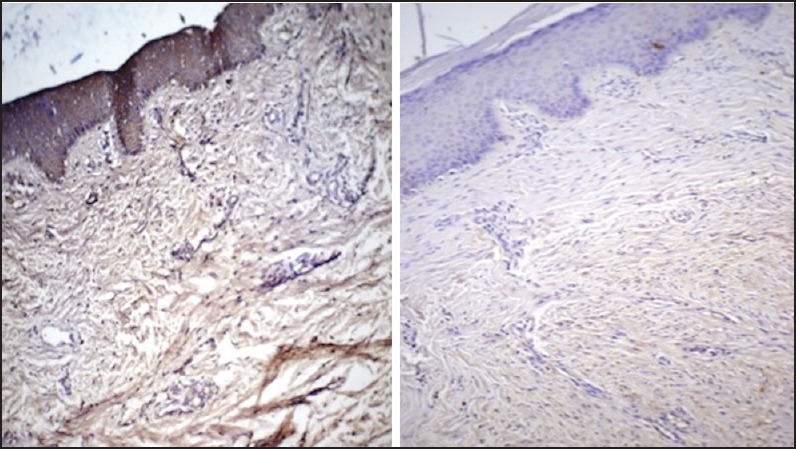
| Fig. 4 Scar tissue stained with anti-transforming growth factor-brta (TGF-β1) (left side picture) and anti-TGF-β2 (right side picture). There is diffuse expression of TGF-β1 throughout the tissue in comparison to TGF-β2 (original magnification, ×10)
Connective tissue
In the normal oral mucosa expression of TGF-β1 was located to the submucosa and superficial muscle collagen with deeper layers of muscle showing sparse positivity. In comparison, TGF-β2 was found to be expressed more in the muscle and peri-muscular areas. The expression of both the biomarkers was the least in the submucosa in comparison with the other groups, a feature found to be statistically significant [Table 3].
Table 3
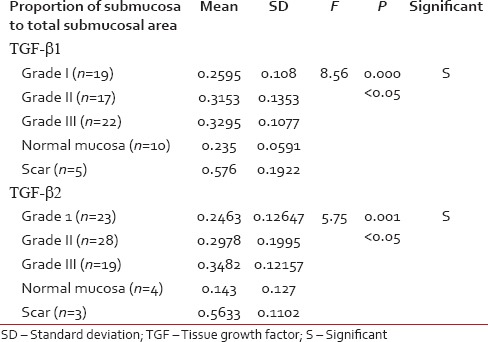
Proportion of TGF-β1 and TGF-β2 in the submucosa to total submucosal area
In Grade I OSF, TGF-β1 expression was present in the submucosal region and muscle. The TGF-β2 expression, in contrast, was located in the submucosa with no involvement of the muscle and mild ingress into the perimuscular fibres. Expression of TGF-β1 was almost 3 times that of TGF-β2 in the muscle region [Table 4]. The propensity of the muscle expression of TGF-β1 was backed statistically [Table 5 and Figure 1].
Table 4
Proportion of TGF-β1 and TGF-β2 in the muscle to total muscle area
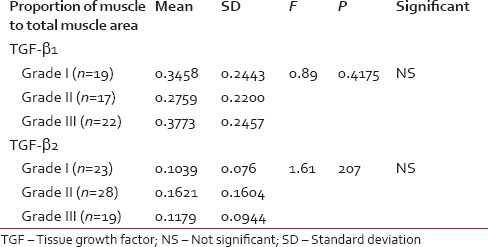
Table 5
Statistical comparison (Student's t-test) of expression of TGF-β1 and TGF-β2 in the various tissues
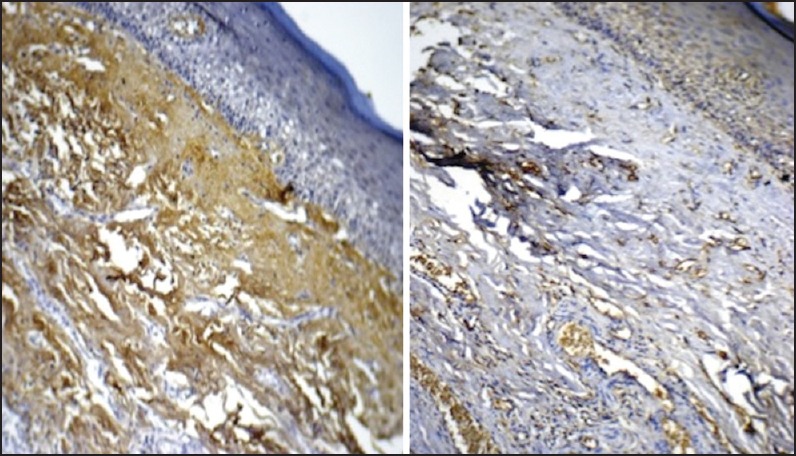
 In Grade II OSF, expression of TGF-β1 paralleled the course seen in Grade I, albeit with increased area of tissue being involved. Thus both submucosa and muscle showed equal expression of the biomarker. In similar contrast, TGF-β2 expression was minimal in the muscle layer, although expression in the perimuscular collagen was prominent. Expression of TGF-β1 in the submucosa and muscle was almost twice that of TGF-β2 and was found to be statistically significant [Figure 2].
In Grade II OSF, expression of TGF-β1 paralleled the course seen in Grade I, albeit with increased area of tissue being involved. Thus both submucosa and muscle showed equal expression of the biomarker. In similar contrast, TGF-β2 expression was minimal in the muscle layer, although expression in the perimuscular collagen was prominent. Expression of TGF-β1 in the submucosa and muscle was almost twice that of TGF-β2 and was found to be statistically significant [Figure 2].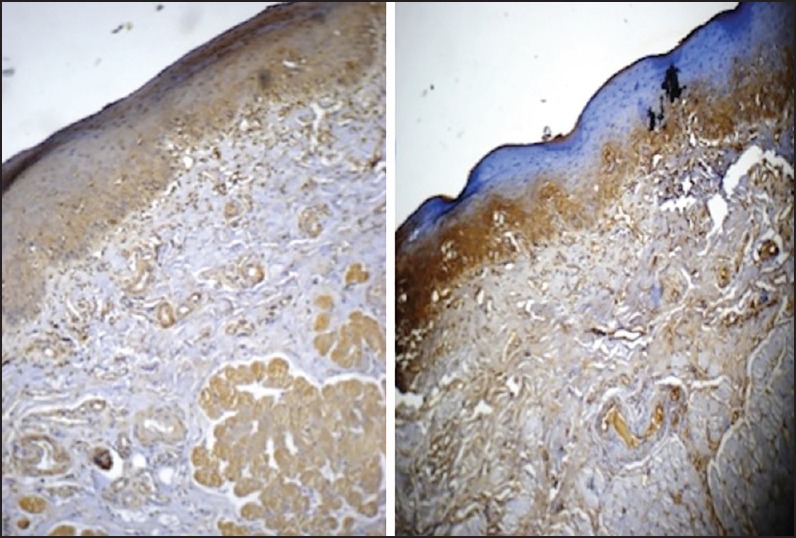
| Fig. 2 Grade II oral submucous fibrosis stained with antitransforming growth factor-beta1 (TGF-β1) (left side picture) and anti-TGF-β2 (right side picture). Note restriction of TGF-β2 expression to submucosa, especially subepithelial (original magnification, ×10)
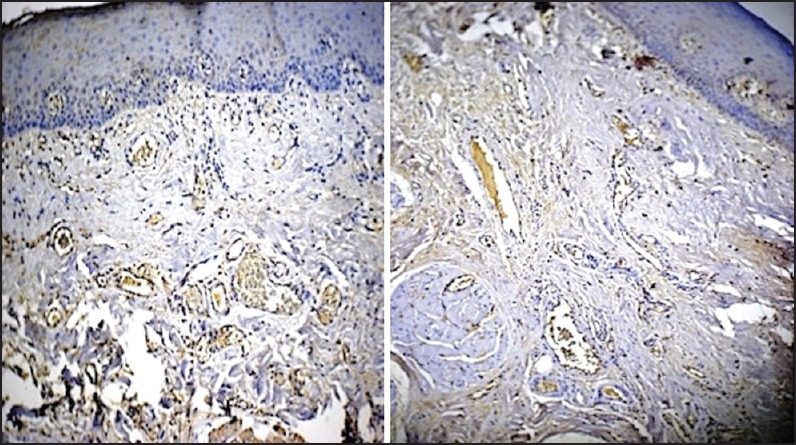
In Grade III OSF, overall increase in the areas positive for TGF-β1 were seen. The expression, in terms of area of tissue, was the highest quantified in all the groups assessed, and 3 times more than TGF-β2. This expression was the most statistically significant amongst all groups of OSF studied [Figure 3].
| Fig. 3 Grade III oral submucous fibrosis stained with antitransforming growth factor-beta1 (TGF-β1) (left side picture) and anti-TGF-β2 (right side picture). The stark contrast in the intensities of expression of the two cytokines is glaringly evident (original magnification, ×10)
Scar tissue, with its established collagen deposition was an ideal comparative subject in the study. Quantified expression of both the antibodies was almost equal and the highest among all groups. Unfortunately due to superficial depth of the biopsies muscle evaluation was not possible in this group [Figure 4].
DISCUSSION
Oral submucous fibrosis is a chronic inflammatory fibrotic disease and a well-established potentially malignant condition of the oral cavity.[5] The disorder is causatively linked to the consumption of areca nut, a widespread habit with social linkages, in the Indian subcontinent. Approximately 5 million people (0.5% of the Indian population of India) are afflicted by OSF. It is of great alarming concern since youngsters are increasingly being affected by the disorder due to the increasing popularity of gutkha (areca nut with tobacco) and pan masala (powdered areca nut with additives but not tobacco) chewing habit in their peer group. With a rate of malignant transformation of 7.6%,[6] the highest amongst the group of potentially malignant oral disorders, the demands on the health care system in treatment of afflicted individuals cannot be understated. The morbidity and debility caused by the disorder is in itself a challenge to the oral health workers.
The chronic micro-trauma due to the chewing and liberation of the contents of arecanut leads to dense inflammation and juxtrepithelial fibrosis of the oral mucosa. TGF-β plays a prominent role in the pathogenesis of OSF, particularly TGF-β1, by ECM mediated increased deposition of collagen and its decreased degradation. Here TGF-β plays a dual role of stimulation as well as inhibition.
Transforming growth factor-beta isoforms exhibit overlapping but distinct temporal and spatial patterns of expression in vivo. TGF-β is a key mediator of tissue fibrosis resulting from accumulation of extra cellular matrix activator proteins, inducing transcription of COL1A1 procollagen gene, increasing levels and activities of the N-and C-procollagen proteinases and promoting the expression of lysyl oxidases. The latter is an essential enzyme for final processing of collagen fibers into a stabilized covalently cross-linked mature fibillar form that is resistant to proteolysis. It decreases the collagen degradation by activating tissue inhibitor of matrix metalloproteinase gene and plasminogen activator inhibitor gene. TGF-β causes induction of connective tissue growth factor, which further mediates stimulatory actions of TGF-β on ECM synthesis.[1]
The present study aimed to identify and correlate the expression of TGF-β1 and TGF-β2 immunohistochemically in different grades of OSF and also to compare its expression with normal oral mucosa and scar tissue.
Transforming growth factor-beta1 and β2 showed intense positivity for OSF and scar tissue whereas mild to moderate reactivity was seen in normal oral mucosal tissue. This is consistent with previously reported studies.[7,8,9,10]
Expression was noted in all the layers of the epithelium, except the keratinized layer. Staining of TGF-β1 was found to be more intense in the spinous layer and its intercellular junctions than in the basal and parabasal layer. This observation does not seem to be reported in literature. It is plausible that prominent intercellular spacing of epithelial cells in the spinous layer enhanced the visual acuity. The expression in terms of tissue area was more in scar tissue and almost equivalent in all grades of OSF and normal oral mucosa. It is well established that inflammatory changes in early stages of OSF stimulate keratinocytes to produce TGF-β1 and β2. The subsequent passage of the cytokines to the connective tissue initiate changes that result in the pathognomic fibrosis of the disorder.[8,11] TGF-β2 expression followed a similar pattern, with decreased intensity and reduced area of involvement.
In the connective tissue, amongst the cellular compartment, fibroblasts, inflammatory cells and endothelial cells expressed the cytokines. The localization of the cytokines to these cells has previously been reported.[12,13] It has also been suggested that elevated expression of cytokines in OSF may be remarked as a signature of the wound healing process.[7]
Transforming growth factor-beta1 was expressed prominently in muscle fibroblasts in contrast to TGF-β2. The latter cytokine is associated with muscle tissue during myogenesis and its presence in mature muscle is minimal. On the other hand TGF-β1 is known to be associated with postinflammatory muscle repair and its expression in OSF and scar is attributable to attempts at the same.[9] In studies carried out on muscle in OSF, it was observed that the muscle tissue is invaded by cytokines probably leading to deposition of mature collagen fibers in the advanced stages of OSF. Due to strain and trauma of the fibrosis around the striated muscle, pathological changes like necrosis are initated.[14] This signals repair and the ingress of TGF-β1 cytokine in the area. The cycle of irritation-inflammation-repair-fibrosis is the hallmark of OSF and not much dissimilar to that occurring in the rest of the body. TGF-β1 has been used as a marker for injured muscle[15] and the minimal expression of TGF-β2 in the present study may be suggestive of formation of myofibrils as regenerative mechanism of the damaged muscle.
The ubiquitous presence of TGF-β1 and the parallel, albeit reduced, expression of TGF-β2 point to the involvement of these cytokines in the pathogenesis of the lesion. Quantitatively and qualitatively, TGF-β1 seems to be the prominent cytokine in modulation of the fibrosis with TGF-β2 playing a “tag-along” role. The role of TGF-β2 has previously been evaluated in OSF. In a study on cultured human keratinocyte cell lines (HaCaT cells) and periodontal fibroblasts (PDC cells), arecoline was found to induce the expression of TGF-β2 mRNA while TβRII expression was downregulated. Over expression of TGF-β2 was also observed in most of OSF tissues compared to normal oral mucosa. Based on these observations the authors feel a definitive involvement of TGF-β2 in the OSF disease process. Investigating the possible mode of action of this cytokine at the cellular level, they found that stimulation of the M3 muscarinic receptor by arecoline leads to the expression of TGF-β2 in the tissues.[16]
The present study has attempted to map the expression of the two cytokines in the tissues in an attempt to outline the pathogenesis of the disease. TGF-β1 was expressed in the epithelium, submucosa and muscle tissue whereas TGF-β2 showed restricted expression to submucosa and superficial muscle areas. The intensity of expression and area occupied by TGF-β1 was more than TGF-β2 and progressively increased with advancing grades of the disease. The expression of the two cytokines was lowest in normal oral mucosa, while in some regions of scar tissue more than that of OSF. It does seem that the induction of TGF-β1 and TGF-β2 is arecoline-mediated and both the epithelial keratinocytes and connective tissue cells are capable of production of the two cytokines. The target of action seems to be primarily the collagen in the submucosa with muscle getting involved only in case of need of repair. Involvement of TGF-β2 in the latter is probably minimal.
The development of fibrosis in OSF is rather complicated and involves an interplay of various ECM components where the balance between production and degradation tilts in favour of the former. That the TGF-β pathway is the most important one in the pathogenesis of the disorder is increasingly being recognized.[7,8,9,10,11,17,18] Amongst the various isoforms of the cytokine β1and β2 seem to be the only isoforms involved in the development of fibrosis.
The present study conclusively demonstrates the expression of TGF-β1 and TGF-β2 in OSF tissues of various stages. The relationship in terms of intensity of expression and tissue area occupied certainly seems to indicate that the actions of both the cytokines is synergistic in OSF. It would be interesting to target therapeutic interventions at this node with the intention of either preventing or arresting the fibrosis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES

| Fig. 1 Grade I oral submucous fibrosis tissue stained with antitransforming growth factor-beta1 (TGF-β1) (left side picture) and anti-TGF-β2 (right side picture). Note intensity and extent of TGF-β1 expression especially in the deeper layers and epithelium (original magnification, ×10)

| Fig. 4 Scar tissue stained with anti-transforming growth factor-brta (TGF-β1) (left side picture) and anti-TGF-β2 (right side picture). There is diffuse expression of TGF-β1 throughout the tissue in comparison to TGF-β2 (original magnification, ×10)


 PDF
PDF  Views
Views  Share
Share

