Vitamin D insufficiency among children with cancer in India
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2016; 37(01): 14-19
DOI: DOI: 10.4103/0971-5851.177009
Abstract
Background: Vitamin D plays an important role in regulating various homeostatic mechanisms and has yet untapped potential in cancer prevention and prognosis. Only a few studies have been done worldwide in relating the Vitamin D levels in pediatric cancer patients to the general population but none so far in an Indian setting to the best of our knowledge. Objective: To compare the Vitamin D levels in a group of children with cancer to that of the general pediatric population and to note differences in the prevalence of Vitamin D insufficiency and make inferences arising from demographic and therapeutic variations. Materials and Methods: Vitamin D levels were found by immuno-chemilumino-metric assay in 102 children (51 cases and 51 controls) over a 6 months period. Results: In comparing the Vitamin D levels of children with cancer and controls from a healthy population we found an increased incidence of Vitamin D insufficiency in cancer children (80.39%) when compared to controls (50.98%) and a much lower mean Vitamin D value in cancer children (22.8 ng/ml) when compared to controls (33 ng/dl). It was also found that cancer children above 6 years had a greater chance for developing Vitamin D insufficiency (P = 0.038) as did children suffering from hematological malignancies (P = 0.025). Conclusion: Our study showed an increased prevalence of Vitamin D insufficiency in children with cancer and hence we suggest routine measurement of Vitamin D levels in children with cancer and subsequent supplementation.
Publication History
Article published online:
12 July 2021
© 2016. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:
Vitamin D plays an important role in regulating various homeostatic mechanisms and has yet untapped potential in cancer prevention and prognosis. Only a few studies have been done worldwide in relating the Vitamin D levels in pediatric cancer patients to the general population but none so far in an Indian setting to the best of our knowledge.
Objective:
To compare the Vitamin D levels in a group of children with cancer to that of the general pediatric population and to note differences in the prevalence of Vitamin D insufficiency and make inferences arising from demographic and therapeutic variations.
Materials and Methods:
Vitamin D levels were found by immuno-chemilumino-metric assay in 102 children (51 cases and 51 controls) over a 6 months period.
Results:
In comparing the Vitamin D levels of children with cancer and controls from a healthy population we found an increased incidence of Vitamin D insufficiency in cancer children (80.39%) when compared to controls (50.98%) and a much lower mean Vitamin D value in cancer children (22.8 ng/ml) when compared to controls (33 ng/dl). It was also found that cancer children above 6 years had a greater chance for developing Vitamin D insufficiency (P = 0.038) as did children suffering from hematological malignancies (P = 0.025).
Conclusion:
Our study showed an increased prevalence of Vitamin D insufficiency in children with cancer and hence we suggest routine measurement of Vitamin D levels in children with cancer and subsequent supplementation.
INTRODUCTION
Vitamin D and its numerous implications on health have been a major field of research in the preceding decades, and the results from these studies have been found to be revelatory, prompting the need for routine Vitamin D level assays in both the general population and among children with cancer. Vitamin D levels have been implicated in the occurrence of a wide variety of diseases such as asthma,[1,2,3] diabetes,[4,5,6] heart disease,[5,7] and cancer[6] where it was once thought the ultimate manifestation of Vitamin D deficiency was rickets or osteomalacia.[8,9,10] Although several studies have been done internationally on this topic,[11,12,13,14] none has been carried out thus far in an Indian population. The aim of our study was to find the prevalence of Vitamin D insufficiency in children with cancer and once found to compare it with the prevalence of the same among the general population and also to compare Vitamin D levels based on characteristics such as age, sex, duration of therapy, and type of cancer.
MATERIALS AND METHODS
Study population and inclusion criteria
Vitamin D concentration in serum was measured from 51 cases and 51 controls over a span of 5 months from March 2013 to July 2013 in our center. Inclusion criteria for subjects were children aged 0-18 years previously or newly diagnosed with malignancy and on treatment whose Vitamin D levels were measured at least once during admission. Only the first measurement of 25-hydroxyvitamin D (25-OH D) was considered and children receiving supplementation were excluded from the study. Exclusion criteria also included children of therapy for a period >2 years, patients suffering from a disease that appeared due to Vitamin D deficiency and patients above 18 years of age at diagnosis. Approval for the study was obtained from the Institute's Ethics Committee.
Data collection
Demographic data collected from our subjects included age and sex. Age was recorded in years and categorized into two groups, those below 6 years and those above 6 years as seen in healthy populations.[15,16,17] Data were also collected on two other criteria and patients divided into two categories based on the type of malignancy as either diagnosed with leukemia/lymphoma or solid tumors and also the duration of therapy.
Assessment of Vitamin D levels
25-OH Vitamin D levels were assessed by immuno-chemilumino-metric assay. Blood samples measuring at least 3 ml were stored in an ice box and sent to the laboratory within 1 h of collection of the sample. 25-OH Vitamin D levels were chosen over 1,25-OH Vitamin D levels since Vitamin D deficiency leads to increase in parathyroid hormone levels, which increase the activity of the enzyme 1α-hydroxylase that in turn affects 1,25-OH Vitamin D levels.[18,19] In addition 1,25-OH Vitamin D has a half-life of only 4 h as opposed to 25-OH Vitamin D with a half-life of 2-3 weeks.[18,19] The cut-off value to define Vitamin D insufficiency was <30 ng/ml as decided by previous studies.[16,20]
Statistical analysis
The main objective of our study was to establish the prevalence of Vitamin D insufficiency in children with cancer. The collected data were analyzed with SPSS for Windows, Version 16.0, SPSS Inc., Chicago, IL, USA. Percentage analysis was used for categorical variables and for continuous variables the mean and standard deviation was used. To find the significant difference between the bivariate samples in independent groups the unpaired t-test was used and to find the significance in categorical data Chi-square test was used. In all the above statistical tools 0.05 was set as the probability value below which results were considered significant.
RESULTS
The Vitamin D levels of 51 patients with malignancy and 51 controls were collected using the method described above. The different characteristics on which the patients were segregated have been described in Table 1. The median age of our patient group was 9.2 years. The majority of patients had either leukemia or lymphoma, but patients with solid tumors were also assessed. All of our patients were on therapy at the time of measurement but were segregated into two groups based on the duration of therapy as less than or more than 1 year of therapy.
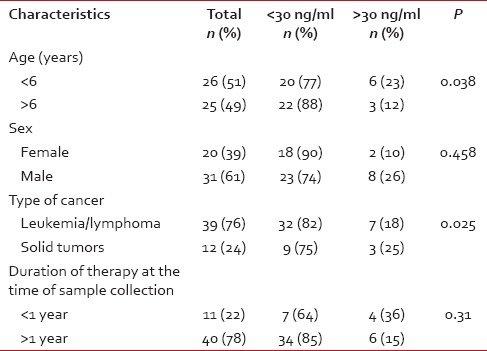
Table 1
Characteristics of the study population by 25-hydroxyvitamin D levels (n = 51)
Vitamin D insufficiency in children with cancer
In our study, we observed that the prevalence of subjects with Vitamin D insufficiency was 80.39% whereas the prevalence of Vitamin D insufficiency in healthy controls was 50.98%. When segregated into two groups based on age it was found that the prevalence in children < 6 years of age was 73% and in children above 6 years of age was 88%.
When segregated into groups based on level of Vitamin D deficiency, 5.8% of subjects were considered severely deficient, i.e., having 25-OH D < 10 ng/ml, 45.1% of subjects were considered deficient, i.e., having 25-OH D >10 but < 20 ng/ml, 29.4% of subjects were considered insufficient, i.e., having 25-OH D > 20 but <30 ng/ml and 19.6% of subjects had adequate levels of Vitamin D, i.e., > 30 ng/ml. It was also found that the prevalence was greater in girls at 90% as compared to boys with 74%. The prevalence of leukemia/lymphoma cases was 82% and in children with solid tumors was 75%. Patients were also divided into two groups based on the duration of therapy at the time of measurement as either receiving therapy for less than a year or more than 1. This is shown in Table 1.
The comparison of Vitamin D levels in healthy subjects versus cancer patients is shown in Figure 1 and the distribution of Vitamin D levels among cases is shown in Table 2.
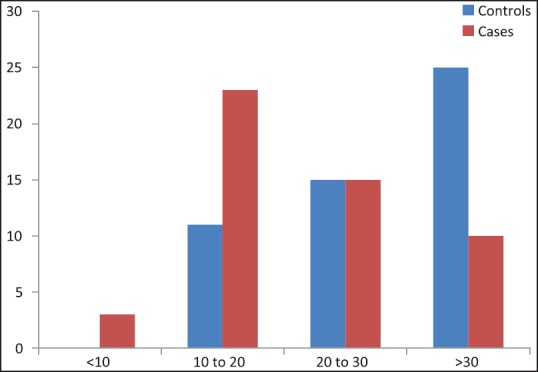
| Fig. 1 Comparison of Vitamin D levels in healthy subjects versus cancer patients (values in ng/ml) (original)
Table 2
Distribution of 25-hydroxyvitamin D levels in the study population (n = 51)

DISCUSSION
The problem of Vitamin D insufficiency and deficiency is widespread in India despite being closer to the equator and the general population having adequate exposure to sunlight. This is perhaps due to a higher percentage of dark skinned people who have an insufficient penetration of ultraviolet radiation needed for Vitamin D synthesis.[20,21]
Pregnant and lactating women have been found to be Vitamin D deficient despite proper supplementation with 73% of women and 80% of children (at birth) being Vitamin D deficient.[22,23,24]
Vitamin D is an essential component of bone and mineral metabolism. Vitamin D is obtained from three sources, sunlight, diet, and dietary supplements.[9,10,18,19] Solar ultraviolet B radiation of wavelength 290-315 nm penetrates skin and converts 7-dehydrocholestrol to pre-Vitamin D3, which is rapidly converted to Vitamin D3.[9] Asian children require 3 times the recommended amount of sunlight exposure to maintain the Vitamin D levels in view of their dark skin color.[20,21] The major dietary sources of Vitamin D are eggs, oily fish, dairy products, and meat. Fortified foods are also an important source of Vitamin D.[25] Fortification of milk has been found to be an effective, safe, and acceptable method,[26] however, in a setting like India, where the per capita milk consumption is very low fortifying milk might not be useful but can be done in salt or cereals. A need for a national food fortification program for Vitamin D in India has been highlighted in a review by Babu and Calvo.[27] It is postulated that pediatric oncology patients are at a risk of developing Vitamin D deficiency in their treatment course due to poor nutrition, lack of sun exposure, and owing to treatment too.[11,14]
Since adequate sunlight exposure at solar noon is difficult to achieve because cancer children spend most of their times indoor, routine oral supplementation may help in preventing Vitamin D deficiency in this group.
Serum levels of Vitamin D are directly related to bone mineral density with optimal density achieved at 40 ng/ml or more[28] Vitamin D deficiency causes growth retardation and skeletal deformities in children and osteomalacia and osteoporosis in adults. Vitamin D3 is converted by 2 hydroxylation steps to the active form. In the liver, Vitamin D3 is converted to 25(OH) D, the major circulating form and in the kidney, 25(OH) D is converted to 1,25-dihydroxyvitamin D (1,25[OH]2D), the biologically active metabolite. Further, 1,25(OH)2D acts by binding to the nuclear Vitamin D receptor (VDR) within cells. This VDR is widely expressed throughout the body. The VDR is found in the endocrine glands such as pituitary, pancreas, parathyroid, gonads, and placenta and in cardiovascular tissues such as endothelial cells, vascular smooth muscle cells, and cardiomyocytes also.[7,29,30] The VDR has also been found in hemato-lymphopoietic cells and also has been shown to regulate cell differentiation and the production of interleukins and cytokines.[21,31] The wide variety of nonskeletal diseases in which Vitamin D plays a role include cardiovascular diseases,[7] obesity,[32] metabolic syndrome,[5] insulin resistance,[4] infection,[33] allergy,[1,2] some forms of cancers,[6,34,35] and autoimmune disease.[1]
It has been found that 1,25(OH)2D decreases cellular proliferation of both normal cells and cancer cells and induces their terminal differentiation.[9,10,36] People living at higher latitudes are at an increased risk of Hodgkin's lymphoma as well as colon, pancreatic, prostate, ovarian, breast, and other cancers and are more likely to die from these cancers, as compared to people living at lower latitudes.[6,34,35]
Both prospective and retrospective epidemiologic studies indicate that levels of 25-OH D below 20 ng/ml are associated with a 30-50% increased risk of prostate, colon, and breast cancer, and melanoma along with increased mortality from these cancers.[35,36,37]
The likely explanation is that colon, prostate, breast, and other tissues express 25-OH D -1α-hydroxylase and produce 1,25(OH)2D locally to control genes that help to prevent cancer by keeping cellular proliferation and differentiation in check.[9,10,38] Children and young adults who are exposed to the most sunlight have a 40% reduced risk of non-Hodgkin's lymphoma[39] and a reduced risk of death from malignant melanoma, as compared to those who have the least exposure to sunlight.[37] It has been postulated that if a cell becomes malignant, 1,25(OH)2D can induce apoptosis and prevent angiogenesis, thereby reducing the potential for the malignant cell to survive.[10] After 1,25(OH)2D completes these tasks, it initiates its own destruction by stimulating the CYP24 gene to produce the inactive calcitroic acid. This guarantees that 1,25(OH)2D does not enter the circulation to influence calcium metabolism.[9,10]
This is a possible explanation for why increased sun exposure and higher circulating levels of 25-OH D are associated with a decreased risk of cancers.[21,38,39]
25(OH)D is the major circulating form of Vitamin D with a half-life of 2-3 weeks, which is the best available indicator of Vitamin D status. Although 1,25(OH)D (calcitriol) is the active form, it has a half-life of only 4 h which is not a good indicator of Vitamin D stores as Vitamin D deficiency can cause PTH elevation that induces increased 1α-hydroxylase activity that results in normal or increased levels of 1,25(OH)D. It also circulates at a concentration that is 100-1000-fold < 25(OH)D.[40]
Data on the prevalence of Vitamin D insufficiency in children are sparse or incomplete in most countries. However, it is an important public health problem in both developed and developing countries, with an estimated prevalence of 29-100% in children and adolescents.[9,41]
There are only a few reports so far that have studied the prevalence of 25-OH D status in cancer survivors.[11,12,13] A prospective cross-sectional study done in the United Kingdom by Sinha et al.[13] comparing 61 cancer survivors aged between 1 and 18 years with 60 controls, found a higher prevalence of 25-OH D deficiency (< 25 ng/ml) among survivors compared to controls, (21.3% in the cancer survivors vs. 3.3% in the controls). The risk factors, which predicted 25-OH D deficiency included seasonality, ethnicity, older age, and a cancer diagnosis.[13]
Simmons et al. studied 78 leukemia survivors, who either received chemotherapy or hematopoietic stem cell transplant and found a prevalence of 25-OH D deficiency (< 15 ng/ml) of 35%. The risk factors found were decreased exposure to sunlight, older age, and lack of 25-OH D supplementation.[12]
Robien et al. in their study in Minnesota looked at 95 cancer survivors aging between 2.7 and 72 years with a diagnosis of leukemia or lymphoma and found the prevalence of 25-OH D deficiency (< 50 ng/ml) to be 10%. Risk factors for 25-OH D deficiency included lack of 25-OH D supplementation and use of steroids.[42]
Even in an Indian context, Vitamin D deficiency has been reported to be present in the majority of children in spite of adequate exposure to sunlight. The prevalence of Vitamin D deficiency is 50-90% in the Indian subcontinent and is attributed to low dietary calcium along with skin color and changing lifestyle.[43,44]
The USA Endocrine Society Guideline recommends screening for Vitamin D deficiency in any population at risk. This includes patients with dark skin who live at a higher altitude and infants born to Vitamin D deficient mothers, infants with poor growth, chronic kidney disease, hepatic failure, malabsorption syndromes, suspected rickets, osteoporosis, inflammatory bowel disease, hyperparathyroidism; those on drug therapy such as anticonvulsants, glucocorticoids, antiretroviral therapy, antifungals, obese children; granuloma-forming disorders such as sarcoidosis, tuberculosis, and histoplasmosis.[45] Currently, they do not recommend cancer survivors or children on chemotherapy for screening, which needs to be considered and revised.
Recommended daily intake of Vitamin D for children, according to the Institute of Medicine is 200 IU a day but experts agree that in the absence of necessary exposure to sunlight, this amount may be increased to 800-1000 IU a day.[46]
The prevalence of Vitamin D insufficiency in our study group (80.39%) was found to be higher than in results obtained from studies done before such as the one done by Sinha et al. where the prevalence of Vitamin D levels below 50 ng/ml in children with cancer was 62%.[13] Despite the study being conducted in a region of higher latitude, the increased prevalence in our subjects may be due to poorer nutritional habits and skin color.
We found out that there was a statistically significant (P = 0.038) increase in the prevalence of Vitamin D insufficiency in children above 6 years (88%) as compared to children below 6 years (77%) that has already been established in healthy populations.[15,16,17] It was also noted that the frequency of severe deficiency was higher in our group when compared to studies done on healthy populations.[15,16,17,47] This is hardly surprising as cancer patients are exposed to less sunlight since they are discouraged from playing outside for sanitary reasons have a poorer nutritional status and are on therapy that is potentially bone weakening.
The prevalence was greater in girls at 90% as compared to boys with 74% but was found to be statistically insignificant, perhaps due to the small size of our sample.
The prevalence of leukemia/lymphoma cases (82%)was significantly (P = 0.025) higher than in children with solid tumors (75%) that went against the study conducted by Helou et al. where prevalence was higher in children suffering from solid tumors,[48] perhaps due to the more intense chemotherapy that these patients receive and greater use of steroids in therapy.
When segregated based on the duration of therapy received there was no significant difference between children receiving therapy for less than a year compared to those receiving therapy for greater than a year and this may suggest that Vitamin D insufficiency may already set in during the beginning stages of therapy.
The limitations of our study were that we had a small sample size, pretreatment and posttreatment comparison of Vitamin D levels was not done which would give a more accurate analysis of effect of treatment on Vitamin D levels in cancer patients and the samples were all collected in a span of 6 months, thereby failing to note the effect of seasonal variation on Vitamin D levels.
CONCLUSION
We have only just begun to understand the role of Vitamin D in homeostatic pathways. Where once it was thought rickets and osteomalacia were the apex of Vitamin D deficiency, we now realize that it is just one of many manifestations of Vitamin D deficiency/insufficiency. Our study shows evidence of increased incidence of Vitamin D insufficiency in children with cancer and as such suggest Vitamin D assays and subsequent supplementation be made a part of the initial tests run at diagnosis in children with cancer. It has also been proved conclusively that patients with certain types of cancer have an increased frequency of VDR genotype variants.[29,30] A future area where the role of Vitamin D may be further studied is in determining VDR polymorphisms in patients with cancer and its role in determining Vitamin D levels and disease progression.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES

| Fig. 1 Comparison of Vitamin D levels in healthy subjects versus cancer patients (values in ng/ml) (original)


 PDF
PDF  Views
Views  Share
Share

