Clinicopathological Profile of Anaplastic Lymphoma Kinase-positive Nonsmall Cell Lung Cancer: An Indian Perspective
CC BY-NC-ND 4.0 ? Indian J Med Paediatr Oncol 2018; 39(01): 32-35
DOI: DOI: 10.4103/ijmpo.ijmpo_19_17
Abstract
Background:?A novel fusion gene of echinoderm microtubule-associated protein-like 4 (EML4) and anaplastic lymphoma kinase (ALK) has been identified in a subset of non-small-cell lung cancers (NSCLCs). Patients with the ALK-EML4 fusion gene demonstrate unique clinicopathological and physiological characteristics. Here we present an analysis of clinicopathological profile of patients of metastatic adenocarcinoma harboring the ALK-EML4 fusion gene.?Methods:?A retrospective analysis of advanced ALK positive NSCLC, who presented at this tertiary care hospital of armed forces from September 2014 to December 2016 was conducted. The primary goal was to evaluate demographic and clinicopathological profile of ALK positive advanced NSCLC. Detection of ALK fusion was done by IHC on formalin fixed paraffin embedded cell blocks.?Results:?Out of 270 patients of NSCLC, 15 (7.4%) tested positive for ALK-EML4 fusion. Rate of positivity was higher in females (13.7%) than in males (5%). The correlation of the ALK-EML4 fusion gene and clinicopathological characteristics of NSCLC patients demonstrated a significant difference in smoking status, histological types, stage, and metastatic pattern.?Conclusion:?Our analysis indicated that ALK-EML4 positive NSCLC comprised a unique subgroup of adenocarcinomas with distinct clinicopathological and radiological characteristics. Incidence of ALK positivity was found to be higher in females and never smokers. These patients have distinct pathological and radiological characteristics.
Keywords
Adenocarcinoma - anaplastic lymphoma kinase-echinoderm microtubule-associated protein-like 4 - fluorescent in situ hybridization - immunohistochemistry - metastasis - nonsmall cell lung cancer
Publication History
23 June 2021 (online)
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:?A novel fusion gene of echinoderm microtubule-associated protein-like 4 (EML4) and anaplastic lymphoma kinase (ALK) has been identified in a subset of non-small-cell lung cancers (NSCLCs). Patients with the ALK-EML4 fusion gene demonstrate unique clinicopathological and physiological characteristics. Here we present an analysis of clinicopathological profile of patients of metastatic adenocarcinoma harboring the ALK-EML4 fusion gene.?Methods:?A retrospective analysis of advanced ALK positive NSCLC, who presented at this tertiary care hospital of armed forces from September 2014 to December 2016 was conducted. The primary goal was to evaluate demographic and clinicopathological profile of ALK positive advanced NSCLC. Detection of ALK fusion was done by IHC on formalin fixed paraffin embedded cell blocks.?Results:?Out of 270 patients of NSCLC, 15 (7.4%) tested positive for ALK-EML4 fusion. Rate of positivity was higher in females (13.7%) than in males (5%). The correlation of the ALK-EML4 fusion gene and clinicopathological characteristics of NSCLC patients demonstrated a significant difference in smoking status, histological types, stage, and metastatic pattern.?Conclusion:?Our analysis indicated that ALK-EML4 positive NSCLC comprised a unique subgroup of adenocarcinomas with distinct clinicopathological and radiological characteristics. Incidence of ALK positivity was found to be higher in females and never smokers. These patients have distinct pathological and radiological characteristics.
Keywords
Adenocarcinoma - anaplastic lymphoma kinase-echinoderm microtubule-associated protein-like 4 - fluorescent in situ hybridization - immunohistochemistry - metastasis - nonsmall cell lung cancer
Introduction
For a long time, systemic cytotoxic chemotherapy remained the gold standard in the treatment of advanced nonsmall cell lung cancer (NSCLC) patients. With the discovery of the anaplastic lymphoma kinase (ALK) gene in NSCLC and the development of crizotinib against this subset of NSCLC, an era of targeted therapies took the lead way.[1],[2] The echinoderm microtubule-associated protein-like 4 (EML4) ? ALK is a fusion-type protein tyrosine kinase, found in 4%?5% of NSCLC.[3] The ALK gene arrangements are largely mutually exclusive with epidermal growth factor receptor (EGFR) or Kirsten ras mutations.[4] Immunohistochemistry (IHC), fluorescent?in situ?hybridization, and reverse transcription polymerase chain reaction have been used to detect ALK mutation. The presence of an ALK fusion oncogene defines a molecular subset of NSCLC with distinct clinical and pathological features. This subset of patients are characterized by relatively younger age, nonsmokers or light smokers, and a mucinous, cribriform, or signet-ring cell subtype of adenocarcinoma.[5] Whenever possible, therapy of patients with advanced NSCLC should be individualized, based on the molecular and histological features of the tumor. ALK is a straightforward, biology-based biomarker, predicting a high response rate with crizotinib even in heavily pretreated patients and is relatively nontoxic. This study was aimed to analyze data and provide a better understanding of patients with ALK-positive advanced NSCLC.
Objective
To evaluate the epidemiological, clinicopathological profile, and disease characteristics of advanced EML4-ALK-positive NSCLC patients in a tertiary care hospital for armed forces.
Materials And Methods
A retrospective analysis of advanced ALK-positive NSCLC who presented at this tertiary care hospital of armed forces from September 2014 to September 2016 was conducted. The primary goal was to evaluate demographic and clinicopathological profile of ALK-positive advanced NSCLC. Details of these patients were obtained from prospective lung cancer audit database that is maintained in the department of medical oncology. Patients underwent a complete history and physical examination, routine blood testing (complete hemogram, renal and liver function tests). Tumor staging was performed by contrast-enhanced computed tomography chest and abdomen, whole body positron emission tomography-computed tomography, and magnetic resonance imaging brain. Detection of ALK fusion was done by IHC on formalin-fixed paraffin-embedded cell blocks.
Observation
Out of 203 NSCLC patients evaluated, 15 patients (7.3%) tested positive for ALK rearrangements. The median age of ALK-positive NSCLC cases was 39.5 years (range 29?63 years), with a slight female predominance (male: female = 1:1.2) [Table 1]. Smoking history was noted in 20% of all ALK+ cases. There were no female smokers in the study. Thirteen patients (86%) had an Eastern Cooperative Oncology Group performance status 0?1. The most common presentation was cough observed in 73% (11) patients followed by shortness of breath noted in 53% (8) cases [Figure 1]. Hemoptysis and neurological deficits were presenting complaints in 13% of cases each. One patient was a diagnosed case of chronic kidney disease Stage IV on maintenance hemodialysis. Two cases were known hypertensives on antihypertensives. Histologically, adenocarcinoma was the dominant subtype found in 73% (11) of all cases and rest about 26% of them were poorly differentiated carcinoma [Table 2]. Six (55%) of the cases were having mucinous or signet-ring pattern on histology. At baseline, one patient was having Stage III disease with rest 93% (14) were Stage IV disease. Eleven (73%) of the patients had more than 2 sites of metastases, with median number of metastasis equal to three. Most common site of metastases was pleural effusion/pleural deposits (93%, n = 14) followed by brain and bony mets (53%, n = 8) as shown in [Figure 2]. On radio imaging, all the brain metastases were present in anterior cranial fossa. Primary tumor location was central in 66% (n?= 10) whereas peripheral and multiple lesions in 13% (n?= 2) and 20% (n?= 3), respectively.
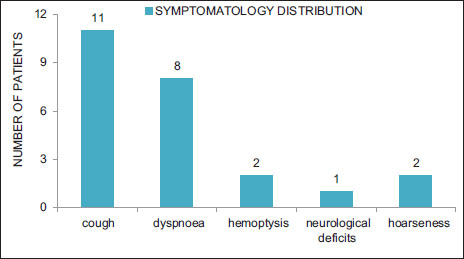
|?Figure.1Symptomatology
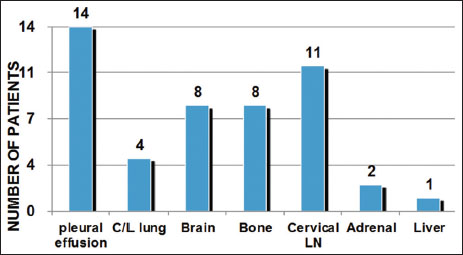
|?Figure.2Sites of metastasis
|
Characteristic |
Number(%) |
|---|---|
|
ALK ? Anaplastic lymphoma kinase; NSCLC ? Nonsmall cell lung cancer; ECOG ? Eastern Cooperative Oncology Group; PS ? Performance status |
|
|
NSCLC patients |
|
|
Study population |
203 |
|
Male:female |
2.7:1 (148/55) |
|
Smoker (all male) (%) |
45 (30) |
|
ALK-positive NSCLC patients |
|
|
Median age (years) |
15/203 (7.3) 39.5 |
|
Male:female |
1:1.2 (7/8) |
|
Smoker/tobacco user (%) |
3 (20) |
|
Comorbidities (%) |
|
|
Hypertension |
2 (13) |
|
Chronic kidney disease |
1 (7) |
|
ECOG PS (%) |
|
|
0-1 |
13 (86) |
|
2 |
1 (7) |
|
>2 |
1 (7) |
|
Clinical features (%) |
|
|
Cough |
11 (73) |
|
Dyspnea |
8 (53) |
|
Hemoptysis |
2 (13) |
|
Hoarseness |
1 (7) |
|
Neurological deficits |
2 (13) |
|
Characteristic |
Number(%) |
|---|---|
|
Histology (%) |
|
|
Adenocarcinoma |
11/15 (73) |
|
Mucinous |
3/11 (27) |
|
Signet ring |
3/11 (27) |
|
Poorly differentiated |
5/11 (45) |
|
Poorly differentiated carcinoma |
4/15 (26) |
|
Stage (%) |
|
|
III |
1 (7) |
|
IV |
14 (93) |
|
Median number of metastatic |
- |
|
sites (%) |
|
|
<2> |
4 (26) |
|
2-4 sites |
11 (73) |
|
>4 sites |
0 |
|
Sites of metastases (%) |
- |
|
Pleural effusion |
14 (93) |
|
Contralateral lung |
4 (26) |
|
Brain |
8 (53) |
|
Bone |
8 (53) |
|
Cervical node |
11 (73) |
|
Adrenals |
2 (13) |
|
Liver |
1 (7) |
|
Primary tumor location (%) |
- |
|
Central |
10 (66) |
|
Peripheral |
2 (13) |
|
Multiple |
3 (20) |
|
Intrathoracic disease (%) |
2 (13) |
|
Intra- and extra-thoracic disease (%) |
13 (87) |

|?Figure.1Symptomatology

|?Figure.2Sites of metastasis
References
- oda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S.?et al.?Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007; 448: 561-6
- unning K, Smith C, Kennedy P, Greenham C.?Examination of the communication interface between students with severe to profound and multiple intellectual disability and educational staff during structured teaching sessions. J Intellect Disabil Res 2013; 57: 39-52
- orn L, Pao W.?EML4-ALK: Honing in on a new target in non-small-cell lung cancer. J Clin Oncol 2009; 27: 4232-5
- akahashi T, Sonobe M, Kobayashi M, Yoshizawa A, Menju T, Nakayama E.?et al.?Clinicopathologic features of non-small-cell lung cancer with EML4-ALK fusion gene. Ann Surg Oncol 2010; 17: 889-97
- ainor JF, Varghese AM, Ou SH, Kabraji S, Awad MM, Katayama R.?et al.?ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS: An analysis of 1,683 patients with non-small cell lung cancer. Clin Cancer Res 2013; 19: 4273-81
- emal A, Bray F, Center MM, Ferlay J, Ward E, Forman D.?et al.?Global cancer statistics. CA Cancer J Clin 2011; 61: 69-90
- emal A, Siegel R, Ward E, Murray T, Xu J, Smigal C.?et al.?Cancer statistics, 2006. CA Cancer J Clin 2006; 56: 106-30
- ttinger DS, Bepler G, Bueno R, Chang A, Chang JY, Chirieac LR.?et al.?Non-small cell lung cancer clinical practice guidelines in oncology. J Natl Compr Canc Netw 2006; 4: 548-82
- iller VA, Kris MG, Shah N, Patel J, Azzoli C, Gomez J.?et al.?Bronchioloalveolar pathologic subtype and smoking history predict sensitivity to gefitinib in advanced non-small-cell lung cancer. J Clin Oncol 2004; 22: 1103-9
- Tiseo M, Gelsomino F, Boggiani D, Bortesi B, Bartolotti M, Bozzetti C.?et al.?EGFR and EML4-ALK gene mutations in NSCLC: A case report of erlotinib-resistant patient with both concomitant mutations. Lung Cancer 2011; 71: 241-3
- d">11?Takeuchi K, Choi YL, Soda M, Inamura K, Togashi Y, Hatano S.?et al.?Multiplex reverse transcription-PCR screening for EML4-ALK fusion transcripts. Clin Cancer Res 2018; 14: 6618-24
- Shaw AT, Yeap BY, Mino-Kenudson M, Digumarthy SR, Costa DB, Heist RS.?et al.?Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 2009; 27: 4247-53
- Shaw AT, Solomon B.?Targeting anaplastic lymphoma kinase in lung cancer. Clin Cancer Res 2011; 17: 2081-6
- Corner J, Hopkinson J, Fitzsimmons D, Barclay S, Muers M.?Is late diagnosis of lung cancer inevitable? Interview study of patients' recollections of symptoms before diagnosis. Thorax 2005; 60: 314-9
- Rodig SJ, Mino-Kenudson M, Dacic S, Yeap BY, Shaw A, Barletta JA.?et al.?Unique clinicopathologic features characterize ALK-rearranged lung adenocarcinoma in the Western population. Clin Cancer Res 2009; 15: 5216-23
- d">16?Yoshida A, Tsuta K, Watanabe S, Sekine I, Fukayama M, Tsuda H.?et al.?Frequent ALK rearrangement and TTF-1/p63 co-expression in lung adenocarcinoma with signet-ring cell component. Lung Cancer 2011; 72: 309-15
- Wong DW, Leung EL, So KK, Tam IY, Sihoe AD, Cheng LC.?et al.?The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer 2009; 115: 1723-33
- Yang P, Kulig K, Boland JM, Erickson-Johnson MR, Oliveira AM, Wampfler J.?et al.?Worse disease-free survival in never-smokers with ALK+ lung adenocarcinoma. J Thorac Oncol 2012; 7: 90-7


 PDF
PDF  Views
Views  Share
Share

