Correlation of Quantitative Diffusion-Weighted MR Parameters and SUVmax from 18-FDG PET-CT in Lung Cancer: A Prospective Observational Study
CC BY 4.0 · Indian J Med Paediatr Oncol 2023; 44(04): 414-421
DOI: DOI: 10.1055/s-0042-1754392
Abstract
Background Diffusion-weighted magnetic resonance imaging (DW-MRI) sequences report the cellularity in tissues and 18-fluorodeoxyglucose (18-FDG) positron emission tomography–computed tomography (PET-CT) provides information on glucose metabolism in cells, associated to tumor aggressiveness. The aim of this study was to assess the correlation between quantitative diffusion-weighted magnetic resonance parameters and maximum standardized uptake value (SUVmax) using 18-FDG PET-CT in lung cancer and metastatic lymph nodes.
Methods Histologically proven 29 patients of lung cancers were subjected to 18-FDG PET-CT and DW-MRI (parameters: repetition time/time to echo [TR/TE] = 4,000/76 ms; b-values = 0, 400, and 800 s/mm2) between June 2018 and June 2019. SUVmax was calculated on the PET-CT images representing region of interest (ROI) in the tumor. The apparent diffusion coefficient (ADC) values were quantified by placing an ROI over the tumor at a high b-value of 800 mm2/s. Statistical analyses for correlation between SUVmax and ADC were done using Pearson's correlation coefficient (r).
Results Significant negative correlation was observed between analyses of ADC and SUVmax for primary lesions of all nonsmall-cell lung cancers (NSCLCs; p < 0.05) and its histological subtype adenocarcinoma (p < 0.05) but not squamous cell carcinomas (p = 0.35). Significant negative correlation was also observed for metastatic lymph nodes of adenocarcinoma (p < 0.05) but not for metastatic lymph nodes of all NSCLCs (p = 0.05) or squamous cell carcinomas (p = 0.55).
Conclusions Diffusion-weighted imaging (DWI) with ADC may represent a new prognostic marker due to a significant negative correlation between ADC determined by DWI and SUVmax by PET-CT in NSCLCs. Furthermore, DWI-MRI of the thorax can be added to routine 18-FDG PET-CT for staging and response assessment in lung cancer in prospects.
Keywords
18-FDG PET-CT - apparent diffusion coefficient (ADC) - nonsmall-cell lung cancer (NSCLC) - SUVmaxPublication History
Article published online:
09 June 2023
© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background Diffusion-weighted magnetic resonance imaging (DW-MRI) sequences report the cellularity in tissues and 18-fluorodeoxyglucose (18-FDG) positron emission tomography–computed tomography (PET-CT) provides information on glucose metabolism in cells, associated to tumor aggressiveness. The aim of this study was to assess the correlation between quantitative diffusion-weighted magnetic resonance parameters and maximum standardized uptake value (SUVmax) using 18-FDG PET-CT in lung cancer and metastatic lymph nodes.
Methods Histologically proven 29 patients of lung cancers were subjected to 18-FDG PET-CT and DW-MRI (parameters: repetition time/time to echo [TR/TE] = 4,000/76 ms; b-values = 0, 400, and 800 s/mm2) between June 2018 and June 2019. SUVmax was calculated on the PET-CT images representing region of interest (ROI) in the tumor. The apparent diffusion coefficient (ADC) values were quantified by placing an ROI over the tumor at a high b-value of 800 mm2/s. Statistical analyses for correlation between SUVmax and ADC were done using Pearson's correlation coefficient (r).
Results Significant negative correlation was observed between analyses of ADC and SUVmax for primary lesions of all nonsmall-cell lung cancers (NSCLCs; p < 0.05) and its histological subtype adenocarcinoma (p < 0.05) but not squamous cell carcinomas (p = 0.35). Significant negative correlation was also observed for metastatic lymph nodes of adenocarcinoma (p < 0.05) but not for metastatic lymph nodes of all NSCLCs (p = 0.05) or squamous cell carcinomas (p = 0.55).
Conclusions Diffusion-weighted imaging (DWI) with ADC may represent a new prognostic marker due to a significant negative correlation between ADC determined by DWI and SUVmax by PET-CT in NSCLCs. Furthermore, DWI-MRI of the thorax can be added to routine 18-FDG PET-CT for staging and response assessment in lung cancer in prospects.
Keywords
18-FDG PET-CT - apparent diffusion coefficient (ADC) - nonsmall-cell lung cancer (NSCLC) - SUVmaxIntroduction
Lung cancer is one of the most common causes of cancer and mortality worldwide. In 2018, 2.1 million new cases (11.6% of the total) and 1.8 million deaths (18.4% of the total) were estimated. The disease remains the most common cancer in men worldwide (14.5% of the total) and the third most common in females (8.4% of the total).[1] Approximately, 80% of lung cancers are nonsmall-cell lung cancers (NSCLCs) and it further has two major types: squamous cell carcinoma and nonsquamous cell carcinoma, including adenocarcinoma and large-cell carcinoma.[2]
NSCLCs diagnosed in the later stages may present with adjoining structures like chest wall/mediastinal invasion, and metastases to lymph nodes or distant organs. The presence of metastases has a significant impact on the disease prognosis and mortality. Early-stage NSCLC cases can be potentially treated by different modalities like surgery/radiotherapy and chemotherapy but advanced or metastatic NSCLC cases are largely incurable.[2] The prognosis of NSCLC depends on many factors like stage, performance status, and molecular markers for treatment regimens.
Targeted therapy is found to be very effective in patients having epidermal growth factor receptor (EGFR) mutations, anaplastic lymphoma kinase (ALK), or ROS1 rearrangements, which are predictive and prognostic markers for NSCLC.[2]
In the last decade, integrated 18-fluorodeoxyglucose (18-FDG) positron emission tomography–computed tomography (PET-CT) was the diagnostic imaging of choice in lung cancer patients for tumor, nodes, and metastases (TNM) staging as it delineates the tumor from adjacent structures anatomically and glucose metabolism in cells by calculating the standardized uptake value (SUV) physiologically.[3] [4] The high SUV in the baseline tumor corresponds to the high glucose metabolism and if the SUV does not show any decrease after initiation of treatment, a poor therapeutic response resulting in lower overall survival rates has been observed.[5] The primary tumor with a high SUV at baseline 18-FDG PET is also associated with lesser duration for progression and more chances of recurrences. Thereby, it makes SUV a crucial prognostic indicator during the course of lung cancer disease.[6]
Magnetic imaging resonance (MRI) with high-performance gradient coils and multiple sequences has paved the way for new sensitive approaches for lung imaging. MRI with 1.5 Tesla and 3 Tesla magnetic field strength sensitively detects lung nodules and lesions and provides morphological details about the tumor without being exposed to ionizing radiation as compared with CT and PET-CT.[7]
Presently, diffusion-weighted magnetic resonance imaging (DW-MRI) is implemented in pulmonary imaging with great prospects in the detection of lung lesions.[8] DW-MRI visualizes the random Brownian motion of molecules within a voxel, which causes incoherent phase shifts resulting in signal attenuation. It helps in quantification of diffusion by measuring the apparent diffusion coefficient (ADC) values in the lesion. Due to high cell density in malignant tumors, water molecules cannot move freely into the interstitial space and show restricted diffusion with lower ADC values. DW-MRI provides information on cellularity in lung cancers, which may have a direct correlation with tumor aggressiveness.[8] [9]
Objective
MRI imaging in lung cancer relatively lacks insight and exposure with no such relevant study ever done in India. With the goal in modern oncology to optimize therapeutic responses and minimize toxicities in patient care, it needs more precise and quantifiable noninvasive parameters for prognosis and early response evaluation to treatment.[10] We aimed to study the correlation between quantitative diffusion-weighted magnetic resonance (MR) parameters and SUVmax using 18-FDG PET-CT in lung cancer.
Materials and Methods
Study Design
This was a prospective observational study conducted at the Rajiv Gandhi Cancer Institute and Research Centre, a tertiary care hospital at New Delhi, after obtaining an Ethics Committee approval.
Study Population
Histopathologically proven 29 patients of lung cancer, who had undergone pretherapeutic staging with 18-FDG PET at all stages, after obtaining an informed consent, were included in this study between June 2018 and June 2019. A sample size of 29 patients was calculated by the formula given below referring to the study done by Tyng et al.[11]
The formula for sample size calculation was:
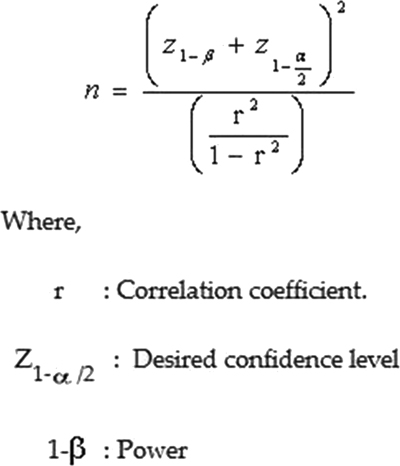
| Figure 1:
where,
correlation coefficient = –0.59215
with power = 95%, n = 24
with power = 90%, n = 19
Z = 5% level of significance
The minimum sample size thus calculated should be 24. Approximate operational sample size = 29 cases.
Patients who underwent any previous antineoplastic treatments like surgery, chemotherapy, or radiation therapy, or contraindicated for MRI (with a cardiac pacemaker, aneurysmal clip, or metal prosthesis) were excluded.
Patients were subjected to 18-FDG PET-CT and brain MRI scans for pretherapeutic staging. For our study, we added thoracic DW-MRI sequences for lung lesions and mediastinal lymph node evaluation.
18-FDG PET-CT Protocol
Precisely, 370 MBq of 18-FDG was injected intravenously 1 hour before the scan. The patient was kept supine and arms held above the head; whole-body examination was performed utilizing a dedicated (Siemens Tru V) system, with four 3.75 mm detectors, 1.5 pitch, and collimation of 5 mm. The CT exposure factors were 140 kVp and 80 mA in 0.8 second. Whole-body PET emission scan was performed, covering an area identical to that of a CT (divided into 5–6 standard bed positions). All acquisitions were performed in a two-dimensional model and consisted of emission scans of 5 minutes per bed position. PET images were reconstructed using CT for attenuation correction by employing CT maps. Transaxial scans of 4.3 × 4.3 × 4.25 mm3 (in-plane matrix size 128 × 128) were reconstructed using OSEM—ordered subsets expectation maximization—with two iterations, 28 subsets, and a filter of 7.0 mm. The axial field of view (FOV) was 148.75 mm, resulting in 35 slices per bed position. Experienced nuclear physicians evaluated the PET-CT images—qualitative (visual) and semiquantitative analyses—with calculation of SUVmax within the lesions. The SUVmax was a representative volumetric region of interest (ROI) in the tumor lesion, normalized to injected dose and patient's weight.
MRI Protocol
Using Siemens Avanto 1.5 Tesla MR Unit, MRI acquisitions were performed by thoracic and body array coil avoiding motion artifacts. Diffusion-weighted imaging (DWI) of thorax was acquired in the axial plane using echo-planar imaging sequence (repetition time/time to echo [TR/TE] = 4,000/76 ms; 5 mm slice thickness; and FOV, 25–30 cm). The b-values used were 0, 400, and 800 mm2/s and ADC maps were generated for all the images. On DWI sequences, the corresponding areas were studied for any restriction and the gray value of the pixel corresponds to the ADC values since a pixel-to-pixel ADC map was automatically calculated for each slice. ADC values were measured manually by placing an ROI over the lesion using pixel-wise ADC maps at a high b-value of 800 mm2/s. The ROI was most representative of the lesion, excluding areas of necrosis, calcifications, or areas that suffer interference or partial volume adjacent to the lesion.
Statistics
Statistical analysis was done using Statistical Package for Social Sciences version 21.0. Categorical variables were presented in number and percentage (%) and continuous variables were presented as mean ± standard deviation (SD) and median.
Normality of data was tested by Kolmogorov–Smirnov test. Quantitative variables were compared using an independent t-test (as the datasets were normally distributed) between the two groups. Pearson correlation coefficient was used for correlation analysis between ADC and SUVmax values. A p-value of <0>
Ethics
The procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1964, as revised in 2013. Ethics Committee approval was obtained from the Institutional Ethics Committee dated August 17, 2018.
Patients were provided patient information sheet regarding study details and an informed consent was obtained prior to enrolment.
Results
Clinical Characteristics of Patients
Overall, 29 patients of NSCLCs—18 males (62.07%) and 11 females (37.9%), range 34 to 86 years with a mean of 61.7 years and a median of 61 years (SD = 11.7 years)—were evaluated. History of smoking was present in 25 cases, that is, 86.21%. In our study, among NSCLCs, adenocarcinoma was the most common histological type (n = 19; 65%), followed by squamous cell carcinoma (n = 10; 34.4%). Details of all the subjects have been provided in [Supplementary Table S1].
Correlation between ADC and SUVmax in NSCLC and Its Histological Variants
We found a statistically significant negative correlation between SUVmax and ADCmin, SUVmax and ADCmean, and SUVmax and ADCmax in all NSCLC cases ([Fig. 1A-C]. Pearson correlation coefficient and p-value are: r = –0.42 and p = 0.02 for correlation between SUVmax and ADCmin; r = –0.39 and p = 0.03 for correlation between SUVmax and ADCmean; and r = –0.42 and p = 0.02 for correlation between SUVmax and ADCmax, respectively.
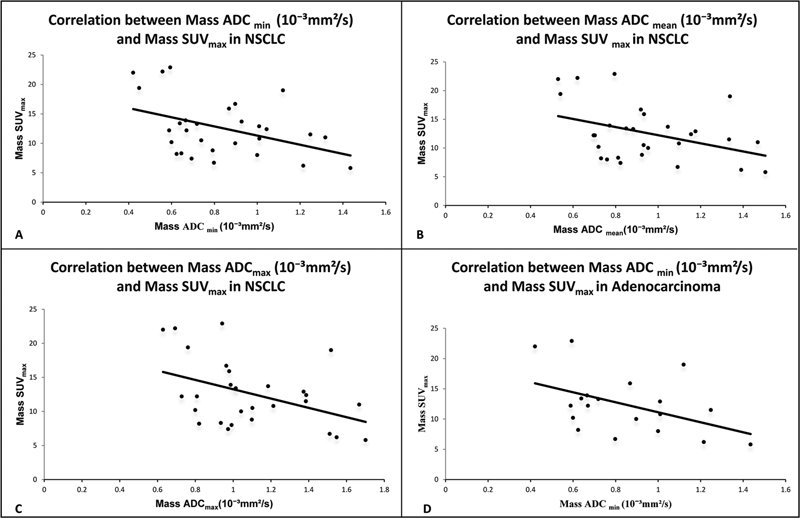
| Figure 1:(A) Correlation between mass ADCmin (10−3 mm2/s) and mass SUVmax in total study subjects with NSCLC. (B) Correlation between mass ADCmean(10−3 mm2/s) and mass SUVmax in total study subjects with NSCLC. (C) Correlation between mass ADCmax (10−3 mm2/s) and mass SUVmax in total study subjects with NSCLC. (D) Correlation between mass ADCmin (10−3 mm2/s) and mass SUVmax in adenocarcinoma. ADC-apparent diffusion coefficient; SUV-standardized uptake value.
[Table 1] shows correlation coefficient (r) and p-value between SUVmax and ADCmin, SUVmax and ADCmean, and SUVmax and ADCmax in all NSCLC cases, and its histological subtypes adenocarcinoma and squamous cell carcinoma. We also found a negative correlation between SUVmax and ADCmin, SUVmax and ADCmean, and SUVmax and ADCmax in all adenocarcinoma cases, a histological variant of NSCLC ([Fig. 1D]). The negative correlation between SUVmax and ADCmin in all adenocarcinoma cases was statistically significant with Pearson correlation coefficient and p-value: r = –0.46 and p = 0.04, respectively.
|
Mass SUVmax |
NSCLC |
Squamous cell carcinoma |
Adenocarcinoma |
|---|---|---|---|
|
Mass ADCmin (10−3 mm2/s) |
|||
|
Correlation coefficient (r) |
–0.423 |
–0.329 |
–0.463 |
|
p-Value |
0.022 |
0.354 |
0.046 |
|
Mass ADCmean (10−3 mm2/s) |
|||
|
Correlation coefficient (r) |
–0.395 |
–0.452 |
–0.364 |
|
p-Value |
0.034 |
0.189 |
0.126 |
|
Mass ADCmax (10−3 mm2/s) |
|||
|
Correlation coefficient (r) |
–0.426 |
–0.479 |
–0.399 |
|
p-Value |
0.021 |
0.161 |
0.091 |
|
Variables |
NSCLC |
Squamous cell carcinoma |
Adenocarcinoma |
|---|---|---|---|
|
Lymph node ADCmin (10−3 mm2/s) |
|||
|
Correlation coefficient (r) |
–0.374 |
0.211 |
–0.522 |
|
p-Value |
0.05 |
0.559 |
0.022 |
|
Lymph node ADCmean (10−3 mm2/s) |
|||
|
Correlation coefficient (r) |
–0.371 |
0.236 |
–0.524 |
|
p-Value |
0.052 |
0.511 |
0.021 |
|
Lymph node ADCmax (10−3 mm2/s) |
|||
|
Correlation coefficient (r) |
–0.306 |
0.264 |
–0.5 |
|
p-Value |
0.113 |
0.461 |
0.029 |
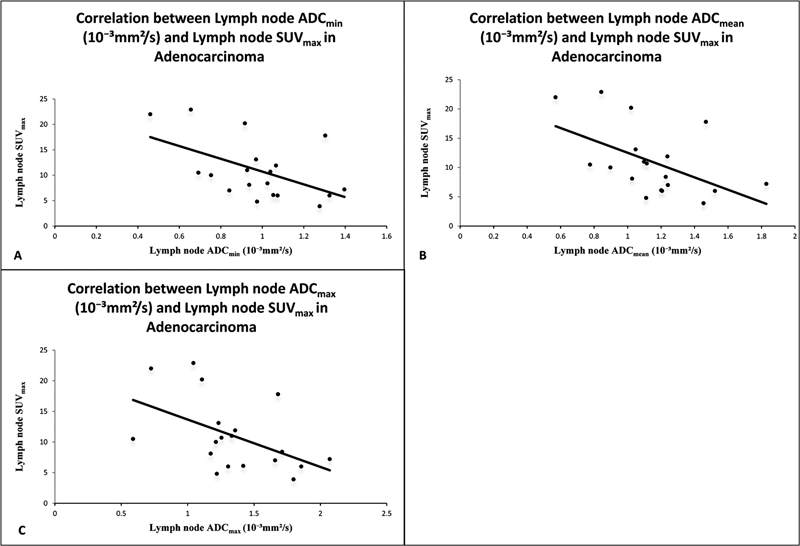
| Figure 2:(A) Correlation between lymph node ADCmin(10−3 mm2/s) and lymph node SUVmax in adenocarcinoma. (B) Correlation between lymph node ADC mean (10−3 mm2/s) and lymph node SUVmax in adenocarcinoma. (C) Correlation between lymph node ADCmax(10−3 mm2/s) and lymph node SUVmax in adenocarcinoma. ADC-apparent diffusion coefficient; SUV-standardized uptake value.
In lymph nodes of squamous cell carcinoma, a negative correlation was found between SUVmax and ADCmin, SUVmax and ADCmean, and SUVmax and ADCmax, but it was not found statistically significant.
Comparison of ADC Values among Lung Mass, Mediastinal Lymph Nodes, and Its Histological Types
In [Supplementary Table S2], the calculated mean ADC values for lung cancers are as follows: 0.83 ± 0.26, 0.95 ± 0.27, and 1.1 ± 0.3 × 10−3 mm2/s (mean ± SD) for ADCmin, ADCmean, and ADCmax, respectively.
The calculated mean ADC values for adenocarcinoma are as follows: 0.85 ± 0.27, 0.96 ± 0.28, and 1.1 ± 0.3 × 10−3 mm2/s (mean ± SD) for ADCmin, ADCmean, and ADCmax, respectively.
The calculated mean ADC values for squamous cell carcinoma are as follows: 0.81 ± 0.26, 0.92 ± 0.27, and 1.0 ± 0.3 × 10−3 mm2/s (mean ± SD) for ADCmin, ADCmean, and ADCmax, respectively.
In [Supplementary Table S3], the calculated mean ADC values for mediastinal lymph nodes in lung cancers are as follows: 0.95 ± 0.29, 1.1 ± 0.34, and 1.3 ± 0.45 × 10−3 mm2/s (mean ± SD) for ADCmin, ADCmean, and ADCmax, respectively.
The calculated mean ADC values for mediastinal lymph nodes in adenocarcinoma are as follows: 0.98 ± 0.24, 1.1 ± 0.29, and 1.36 ± 0.37 × 10−3 mm2/s (mean ± SD) for ADCmin, ADCmean, and ADCmax, respectively.
The calculated mean ADC values for mediastinal lymph nodes in squamous cell carcinoma are as follows: 0.87 ± 0.37, 1.07 ± 0.44, and 1.3 ± 0.59 × 10−3 mm2/s (mean ± SD) for ADCmin, ADCmean, and ADCmax, respectively.
Mean ADC values for adenocarcinoma are slightly higher than ADC values for squamous cell carcinoma in lung cancers and its mediastinal lymph nodes also, but not found statistically significant.
Discussion
Our study comprised of 29 patients of NSCLC and we found a significant negative correlation between ADC and SUVmax for NSCLC, irrespective of its subtypes. This is in congruence with studies by Tyng et al,[11] Regier et al,[12] and Heusch et al[13] who reported a significant negative correlation between ADC and SUVmax variables in 37, 41, and 18 patients of NSCLC, respectively. Therefore, these results explain a direct correlation between the motion of water molecules, cellularity in tissues assessed by DW-MRI, and glucose metabolism in cells evaluated by PET-CT, which are concerned with tumor aggressiveness.
We found statistically significant negative correlation between SUVmax and ADCmin, SUVmax and ADCmean, and SUVmax and ADCmax in all NSCLC cases. Statistically significant negative correlation was also observed between SUVmax and ADCmin in adenocarcinoma cases ([Fig. 3A-D]). We found a negative correlation between SUVmax and ADCmin, SUVmax and ADCmean, and SUVmax and ADCmax in all squamous cell carcinoma cases, but it was not found statistically significant.
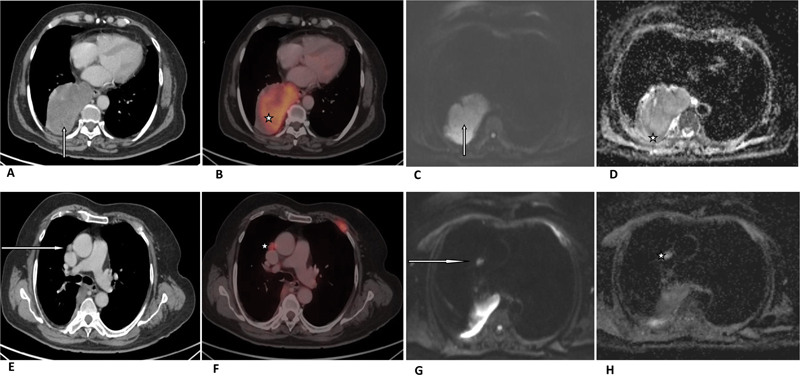
| Figure 3:A 72-year-old patient was diagnosed with adenocarcinoma of the right lung lower lobe with mediastinal lymphadenopathy. (A) Axial CT image in soft tissue window setting (arrow) and (B) corresponding fused PET-CT image are showing right lung lower lobe mass (star) with SUVmax of 15.9. (C) Gray-scale axial DWI of the lower thorax (arrow) and (D) corresponding ADC slice are showing hypointense tumor mass (star) with ADCmax, ADCmean, and ADCmin - 0.97, 0.93, and 0.86 ×10−3 mm2/s, respectively. A 72-year-old patient was diagnosed with adenocarcinoma of the right lung lower lobe with mediastinal lymphadenopathy. (E) Axial CT image in soft tissue window setting (arrow) and (F) corresponding fused PET-CT image are showing right prevascular lymph node (star) with SUVmax of 7.2. (G) Gray-scale axial DWI of the lower thorax (arrow) and (H) corresponding ADC slice are showing hypointense prevascular lymph node (star) with ADCmax ADCmean and ADCmin 2.0, 1.8, 1.39 ×10−3 mm2/s, respectively. ADC- apparent diffusion coefficient; CT-computed tomography; DWI-diffusion-weighted image; PET-positron emission tomography; SUV-standardized uptake value.
PET-CT determines glucose metabolism in the tumor through the activity of 18-FDG by its accumulation in vital cells. An increase in 18-FDG uptake shows an increase in glycolysis due to the high metabolic activity of malignant tumors, the so-called Warburg effect.[14] This gives information on the pathophysiology and growth of the tumor by calculating the SUVmax. In DW-MRI, a decrease in ADC values has been demonstrated in various malignant diseases,[15] tumor characteristics, and the manifestation of lymph node metastases.[16] Hence, both approaches, the SUV determining the metabolic activity on PET-CT and the ADC revealing diffusion restriction due to cellularity in tumor cells, on the other hand, are in direct relation to tumor aggressiveness. These results agree with the hypothesis that DWI may have a role in the imaging evaluation of lung cancers.
The inability of SUV readings to decrease has been linked to a failure to respond to treatment. It is associated with lesser duration for progression and more chances of recurrences with lower overall survival rates.[5] [17] Few studies have demonstrated that low ADC and high SUVmax are associated with poor disease progression after treatment.[18] Iizuka et al[18] evaluated 15 patients of NSCLC with stereotactic body radiotherapy (SBRT). They concluded that a low ADC on pretreatment DW-MRI and a high SUVmax might be associated with poor disease progression in NSCLC patients treated with SBRT, and using both values in combination was a better predictor.
Meanwhile, studies are trying to conclude that DW-MRI may have a better potential for early prediction of early tumor response to therapy and prognosis in advanced lung cancer, and ADC may represent a new prognostic biomarker.[13] [19] [20] [21] Tsuchida et al[22] evaluated 28 patients of advanced lung cancer for response assessment and concluded that DW-MRI could help in prognosis in advanced lung cancer patients. Ohno et al[19] concluded that DWI may have a better potential than 18-FDG PET-CT for prediction of tumor response to therapy in NSCLC patients before chemo-radiotherapy.
Yabuuchi et al[20] and Chang et al[23] showed ADC as a promising tool for monitoring the early response or predicting prognosis after chemotherapy in NSCLC. Until now, tumor response to treatment was determined by a decrease in diameter or size in serial CT studies as chemotherapy causes cell membrane rupture and decrease in cell size and density, which facilitates the diffusion of the molecules after the beginning of the treatment.[24] DW-MRI may evaluate response to treatment earlier, as ADC values may increase before the reduction of tumor size. As in our study, we did not perform DWI-MRI after chemotherapy to see the response assessment in form of change in ADC values in comparison to baseline ADC values. So, we require more studies to study the correlation of ADC and SUV in prognosis and therapeutic response in the population.
Significant negative correlation was observed between SUVmax and ADCmin, SUVmax and ADCmean, and SUVmax and ADCmax in all lymph nodes of adenocarcinoma cases ([Fig. 3E-H]). However, no significant negative correlation was observed in lymph nodes of squamous cell carcinomas. Till now, few studies showed negative correlation between increased glucose metabolism and cellularity in lymph node metastases of NSCLC patients. Schaarschmidt et al[25] compared the ADC in lymph node metastases of NSCLC patients with SUV using 18-FDG PET/MRI in 38 patients and found a weak inverse correlation between SUVmax and ADCmean. Usuda et al[26] found better accuracy of DW-MRI over PET-CT in diagnosing metastatic lymph nodes in NSCLC patients and found a weak negative correlation between SUVmax and ADC. We found that mean ADC values for adenocarcinoma are slightly higher than ADC values for squamous cell carcinoma in lung cancers and its mediastinal lymph nodes also. Matoba et al[8] reported that ADC values are dependent on restricted diffusion within the water microenvironment due to cell membranes, tight junctions, fibers, macromolecules, and cell organelles, and directly related to tumor cellularity and aggressiveness. Therefore, the adenocarcinoma may be having high tumor cellularity due to the microstructural environment that influences ADC values to be higher than the squamous cell carcinoma variant.
There was a significant negative correlation between ADC and SUVmax in NSCLC cases, its histological variant adenocarcinoma, and mediastinal lymph nodes of adenocarcinoma in our study, due to early prediction of tumor response in comparison to PET-CT as described by Yabuuchi et al[20] and Chang et al.[23] So, ADC may represent a new prognostic marker in NSCLC with incremental benefit in staging and response evaluation without radiation exposure.
However, recent study conducted by Bruckmann et al[27] concluded that the combined analysis of SUV and ADC values does not improve the survival prediction in NSCLC and, therefore, ADC values do not further enhance the diagnostic value of SUV as a prognostic biomarker in NSCLC.
Our study has some limitations. The first is the small size of population cohort, and patient selection criteria were biased by inclusion criteria being based on histopathological findings. More studies with a larger cohort and without any potential selection bias are needed.
Conclusion
Our study reveals a significant negative correlation between SUVmax by PET-CT and ADC values by DW-MRI in NSCLC cases and its histological variant, adenocarcinoma. A significant negative correlation is also observed in SUVmax and ADC in metastatic lymph nodes of adenocarcinoma.
DWI with ADC may represent a new prognostic marker due to a significant negative correlation between ADC and SUVmax in NSCLC. Furthermore, DW-MRI of the thorax can be added to routine 18-FDG PET-CT for staging and response assessment in lung cancer in prospects.
Conflict of Interest
None declared.
Acknowledgments
We thank the patients and their families for their munificence in contributing to this study. We would also like to thank all members of the IRB committee who gave their approval for this study.
Supplementary Material
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68 (06) 394-424
- Ettinger DS, Aisner DL, Wood DE. et al. NCCN guidelines insights: non-small cell lung cancer, Version 5.2018. J Natl Compr Canc Netw 2018; 16 (07) 807-821
- De Wever W, Stroobants S, Coolen J, Verschakelen JA. Integrated PET/CT in the staging of nonsmall cell lung cancer: technical aspects and clinical integration. Eur Respir J 2009; 33 (01) 201-212
- Kligerman S, Digumarthy S. Staging of non-small cell lung cancer using integrated PET/CT. AJR Am J Roentgenol 2009; 193 (05) 1203-1211
- Nahmias C, Hanna WT, Wahl LM, Long MJ, Hubner KF, Townsend DW. Time course of early response to chemotherapy in non-small cell lung cancer patients with 18F-FDG PET/CT. J Nucl Med 2007; 48 (05) 744-751
- Borst GR, Belderbos JS, Boellaard R. et al. Standardised FDG uptake: a prognostic factor for inoperable non-small cell lung cancer. Eur J Cancer 2005; 41 (11) 1533-1541
- Regier M, Kandel S, Kaul MG. et al. Detection of small pulmonary nodules in high-field MR at 3 T: evaluation of different pulse sequences using porcine lung explants. Eur Radiol 2007; 17 (05) 1341-1351
- Matoba M, Tonami H, Kondou T. et al. Lung carcinoma: diffusion-weighted MR imaging–preliminary evaluation with apparent diffusion coefficient. Radiology 2007; 243 (02) 570-577
- Takahara T, Imai Y, Yamashita T, Yasuda S, Nasu S, Van Cauteren M. Diffusion weighted whole body imaging with background body signal suppression (DWIBS): technical improvement using free breathing, STIR and high resolution 3D display. Radiat Med 2004; 22 (04) 275-282
- Theilmann RJ, Borders R, Trouard TP. et al. Changes in water mobility measured by diffusion MRI predict response of metastatic breast cancer to chemotherapy. Neoplasia 2004; 6 (06) 831-837
- Tyng CJ, Guimarães MD, Bitencourt AG. et al. Correlation of the ADC values assessed by diffusion-weighted MRI and 18 F–FDG PET/CT SUV in patients with lung cancer. Applied Cancer Research. 2018; 38 (01) 1-7
- Regier M, Derlin T, Schwarz D. et al. Diffusion weighted MRI and 18F-FDG PET/CT in non-small cell lung cancer (NSCLC): does the apparent diffusion coefficient (ADC) correlate with tracer uptake (SUV)?. Eur J Radiol 2012; 81 (10) 2913-2918
- Heusch P, Buchbender C, Köhler J. et al. Correlation of the apparent diffusion coefficient (ADC) with the standardized uptake value (SUV) in hybrid 18F-FDG PET/MRI in non-small cell lung cancer (NSCLC) lesions: initial results. RöFo-Fortschritte auf demGebiet der Röntgenstrahlen und der bildgebendenVerfahren 2013; 185 (11) 1056-1062
- Warburg O. On the origin of cancer cells. Science 1956; 123 (3191): 309-314
- Gourtsoyianni S, Papanikolaou N, Yarmenitis S, Maris T, Karantanas A, Gourtsoyiannis N. Respiratory gated diffusion-weighted imaging of the liver: value of apparent diffusion coefficient measurements in the differentiation between most commonly encountered benign and malignant focal liver lesions. Eur Radiol 2008; 18 (03) 486-492
- Pauls S, Schmidt SA, Juchems MS. et al. Diffusion-weighted MR imaging in comparison to integrated [ 18F]-FDG PET/CT for N-staging in patients with lung cancer. Eur J Radiol 2012; 81 (01) 178-182
- Cerfolio RJ, Bryant AS, Ohja B, Bartolucci AA. The maximum standardized uptake values on positron emission tomography of a non-small cell lung cancer predict stage, recurrence, and survival. J Thorac Cardiovasc Surg 2005; 130 (01) 151-159
- Iizuka Y, Matsuo Y, Umeoka S. et al. Prediction of clinical outcome after stereotactic body radiotherapy for non-small cell lung cancer using diffusion-weighted MRI and (18)F-FDG PET. Eur J Radiol 2014; 83 (11) 2087-2092
- Ohno Y, Koyama H, Yoshikawa T. et al. Diffusion-weighted MRI versus 18F-FDG PET/CT: performance as predictors of tumor treatment response and patient survival in patients with non-small cell lung cancer receiving chemoradiotherapy. AJR Am J Roentgenol 2012; 198 (01) 75-82
- Yabuuchi H, Hatakenaka M, Takayama K. et al. Non-small cell lung cancer: detection of early response to chemotherapy by using contrast-enhanced dynamic and diffusion-weighted MR imaging. Radiology 2011; 261 (02) 598-604
- Yu J, Li W, Zhang Z, Yu T, Li D. Prediction of early response to chemotherapy in lung cancer by using diffusion-weighted MR imaging. Scientific World J 2014; 2014: 135841
- Tsuchida T, Morikawa M, Demura Y, Umeda Y, Okazawa H, Kimura H. Imaging the early response to chemotherapy in advanced lung cancer with diffusion-weighted magnetic resonance imaging compared to fluorine-18 fluorodeoxyglucose positron emission tomography and computed tomography. J Magn Reson Imaging 2013; 38 (01) 80-88
- Chang Q, Wu N, Ouyang H, Huang Y. Diffusion-weighted magnetic resonance imaging of lung cancer at 3.0 T: a preliminary study on monitoring diffusion changes during chemoradiation therapy. Clin Imaging 2012; 36 (02) 98-103
- Dudeck O, Zeile M, Pink D. et al. Diffusion-weighted magnetic resonance imaging allows monitoring of anticancer treatment effects in patients with soft-tissue sarcomas. J Magn Reson Imaging 2008; 27 (05) 1109-1113
- Schaarschmidt BM, Buchbender C, Nensa F. et al. Correlation of the apparent diffusion coefficient (ADC) with the standardized uptake value (SUV) in lymph node metastases of non-small cell lung cancer (NSCLC) patients using hybrid 18F-FDG PET/MRI. PLoS One 2015; 10 (01) e0116277
- Usuda K, Zhao XT, Sagawa M. et al. Diffusion-weighted imaging is superior to positron emission tomography in the detection and nodal assessment of lung cancers. Ann Thorac Surg 2011; 91 (06) 1689-1695
- Bruckmann NM, Kirchner J, Grueneisen J. et al. Correlation of the apparent diffusion coefficient (ADC) and standardized uptake values (SUV) with overall survival in patients with primary non-small cell lung cancer (NSCLC) using 18F-FDG PET/MRI. Eur J Radiol 2021; 134: 109422
Address for correspondence
Publication History
Article published online:
09 June 2023
© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Figure 1:

| Figure 1:(A) Correlation between mass ADCmin (10−3 mm2/s) and mass SUVmax in total study subjects with NSCLC. (B) Correlation between mass ADCmean(10−3 mm2/s) and mass SUVmax in total study subjects with NSCLC. (C) Correlation between mass ADCmax (10−3 mm2/s) and mass SUVmax in total study subjects with NSCLC. (D) Correlation between mass ADCmin (10−3 mm2/s) and mass SUVmax in adenocarcinoma. ADC-apparent diffusion coefficient; SUV-standardized uptake value.

| Figure 2:(A) Correlation between lymph node ADCmin(10−3 mm2/s) and lymph node SUVmax in adenocarcinoma. (B) Correlation between lymph node ADC mean (10−3 mm2/s) and lymph node SUVmax in adenocarcinoma. (C) Correlation between lymph node ADCmax(10−3 mm2/s) and lymph node SUVmax in adenocarcinoma. ADC-apparent diffusion coefficient; SUV-standardized uptake value.

| Figure 3:A 72-year-old patient was diagnosed with adenocarcinoma of the right lung lower lobe with mediastinal lymphadenopathy. (A) Axial CT image in soft tissue window setting (arrow) and (B) corresponding fused PET-CT image are showing right lung lower lobe mass (star) with SUVmax of 15.9. (C) Gray-scale axial DWI of the lower thorax (arrow) and (D) corresponding ADC slice are showing hypointense tumor mass (star) with ADCmax, ADCmean, and ADCmin - 0.97, 0.93, and 0.86 ×10−3 mm2/s, respectively. A 72-year-old patient was diagnosed with adenocarcinoma of the right lung lower lobe with mediastinal lymphadenopathy. (E) Axial CT image in soft tissue window setting (arrow) and (F) corresponding fused PET-CT image are showing right prevascular lymph node (star) with SUVmax of 7.2. (G) Gray-scale axial DWI of the lower thorax (arrow) and (H) corresponding ADC slice are showing hypointense prevascular lymph node (star) with ADCmax ADCmean and ADCmin 2.0, 1.8, 1.39 ×10−3 mm2/s, respectively. ADC- apparent diffusion coefficient; CT-computed tomography; DWI-diffusion-weighted image; PET-positron emission tomography; SUV-standardized uptake value.
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68 (06) 394-424
- Ettinger DS, Aisner DL, Wood DE. et al. NCCN guidelines insights: non-small cell lung cancer, Version 5.2018. J Natl Compr Canc Netw 2018; 16 (07) 807-821
- De Wever W, Stroobants S, Coolen J, Verschakelen JA. Integrated PET/CT in the staging of nonsmall cell lung cancer: technical aspects and clinical integration. Eur Respir J 2009; 33 (01) 201-212
- Kligerman S, Digumarthy S. Staging of non-small cell lung cancer using integrated PET/CT. AJR Am J Roentgenol 2009; 193 (05) 1203-1211
- Nahmias C, Hanna WT, Wahl LM, Long MJ, Hubner KF, Townsend DW. Time course of early response to chemotherapy in non-small cell lung cancer patients with 18F-FDG PET/CT. J Nucl Med 2007; 48 (05) 744-751
- Borst GR, Belderbos JS, Boellaard R. et al. Standardised FDG uptake: a prognostic factor for inoperable non-small cell lung cancer. Eur J Cancer 2005; 41 (11) 1533-1541
- Regier M, Kandel S, Kaul MG. et al. Detection of small pulmonary nodules in high-field MR at 3 T: evaluation of different pulse sequences using porcine lung explants. Eur Radiol 2007; 17 (05) 1341-1351
- Matoba M, Tonami H, Kondou T. et al. Lung carcinoma: diffusion-weighted MR imaging–preliminary evaluation with apparent diffusion coefficient. Radiology 2007; 243 (02) 570-577
- Takahara T, Imai Y, Yamashita T, Yasuda S, Nasu S, Van Cauteren M. Diffusion weighted whole body imaging with background body signal suppression (DWIBS): technical improvement using free breathing, STIR and high resolution 3D display. Radiat Med 2004; 22 (04) 275-282
- Theilmann RJ, Borders R, Trouard TP. et al. Changes in water mobility measured by diffusion MRI predict response of metastatic breast cancer to chemotherapy. Neoplasia 2004; 6 (06) 831-837
- Tyng CJ, Guimarães MD, Bitencourt AG. et al. Correlation of the ADC values assessed by diffusion-weighted MRI and 18 F–FDG PET/CT SUV in patients with lung cancer. Applied Cancer Research. 2018; 38 (01) 1-7
- Regier M, Derlin T, Schwarz D. et al. Diffusion weighted MRI and 18F-FDG PET/CT in non-small cell lung cancer (NSCLC): does the apparent diffusion coefficient (ADC) correlate with tracer uptake (SUV)?. Eur J Radiol 2012; 81 (10) 2913-2918
- Heusch P, Buchbender C, Köhler J. et al. Correlation of the apparent diffusion coefficient (ADC) with the standardized uptake value (SUV) in hybrid 18F-FDG PET/MRI in non-small cell lung cancer (NSCLC) lesions: initial results. RöFo-Fortschritte auf demGebiet der Röntgenstrahlen und der bildgebendenVerfahren 2013; 185 (11) 1056-1062
- Warburg O. On the origin of cancer cells. Science 1956; 123 (3191): 309-314
- Gourtsoyianni S, Papanikolaou N, Yarmenitis S, Maris T, Karantanas A, Gourtsoyiannis N. Respiratory gated diffusion-weighted imaging of the liver: value of apparent diffusion coefficient measurements in the differentiation between most commonly encountered benign and malignant focal liver lesions. Eur Radiol 2008; 18 (03) 486-492
- Pauls S, Schmidt SA, Juchems MS. et al. Diffusion-weighted MR imaging in comparison to integrated [ 18F]-FDG PET/CT for N-staging in patients with lung cancer. Eur J Radiol 2012; 81 (01) 178-182
- Cerfolio RJ, Bryant AS, Ohja B, Bartolucci AA. The maximum standardized uptake values on positron emission tomography of a non-small cell lung cancer predict stage, recurrence, and survival. J Thorac Cardiovasc Surg 2005; 130 (01) 151-159
- Iizuka Y, Matsuo Y, Umeoka S. et al. Prediction of clinical outcome after stereotactic body radiotherapy for non-small cell lung cancer using diffusion-weighted MRI and (18)F-FDG PET. Eur J Radiol 2014; 83 (11) 2087-2092
- Ohno Y, Koyama H, Yoshikawa T. et al. Diffusion-weighted MRI versus 18F-FDG PET/CT: performance as predictors of tumor treatment response and patient survival in patients with non-small cell lung cancer receiving chemoradiotherapy. AJR Am J Roentgenol 2012; 198 (01) 75-82
- Yabuuchi H, Hatakenaka M, Takayama K. et al. Non-small cell lung cancer: detection of early response to chemotherapy by using contrast-enhanced dynamic and diffusion-weighted MR imaging. Radiology 2011; 261 (02) 598-604
- Yu J, Li W, Zhang Z, Yu T, Li D. Prediction of early response to chemotherapy in lung cancer by using diffusion-weighted MR imaging. Scientific World J 2014; 2014: 135841
- Tsuchida T, Morikawa M, Demura Y, Umeda Y, Okazawa H, Kimura H. Imaging the early response to chemotherapy in advanced lung cancer with diffusion-weighted magnetic resonance imaging compared to fluorine-18 fluorodeoxyglucose positron emission tomography and computed tomography. J Magn Reson Imaging 2013; 38 (01) 80-88
- Chang Q, Wu N, Ouyang H, Huang Y. Diffusion-weighted magnetic resonance imaging of lung cancer at 3.0 T: a preliminary study on monitoring diffusion changes during chemoradiation therapy. Clin Imaging 2012; 36 (02) 98-103
- Dudeck O, Zeile M, Pink D. et al. Diffusion-weighted magnetic resonance imaging allows monitoring of anticancer treatment effects in patients with soft-tissue sarcomas. J Magn Reson Imaging 2008; 27 (05) 1109-1113
- Schaarschmidt BM, Buchbender C, Nensa F. et al. Correlation of the apparent diffusion coefficient (ADC) with the standardized uptake value (SUV) in lymph node metastases of non-small cell lung cancer (NSCLC) patients using hybrid 18F-FDG PET/MRI. PLoS One 2015; 10 (01) e0116277
- Usuda K, Zhao XT, Sagawa M. et al. Diffusion-weighted imaging is superior to positron emission tomography in the detection and nodal assessment of lung cancers. Ann Thorac Surg 2011; 91 (06) 1689-1695
- Bruckmann NM, Kirchner J, Grueneisen J. et al. Correlation of the apparent diffusion coefficient (ADC) and standardized uptake values (SUV) with overall survival in patients with primary non-small cell lung cancer (NSCLC) using 18F-FDG PET/MRI. Eur J Radiol 2021; 134: 109422


 PDF
PDF  Views
Views  Share
Share

