Imaging Recommendations for Diagnosis, Staging, and Management of Carcinoma of Unknown Origin (Lymph Node, Pulmonary, Liver, Skeletal, and Brain) with Emphasis on the Current Position of PET-CT in Carcinoma of Unknown Origin (CUP)
CC BY 4.0 · Indian J Med Paediatr Oncol 2023; 44(02): 194-206
DOI: DOI: 10.1055/s-0042-1760311
Abstract
Most of the established guidelines mention and recommend the use of FDG-PET/CT (fluorodeoxyglucose positron emission tomography/computed tomography) in carcinoma of unknown primary (CUP) especially in head–neck squamous cell carcinoma; as described in this article, this forms a powerful one-stop shop in diagnosing and staging modality and has multiple applications in difficult situations of CUPs. Although not used as a screening modality, FDG-PET/CT is recommended as the primary imaging modality in the evaluation of primary, staging, and response evaluation for CUP with histology known to demonstrate FDG avidity, especially patients presenting with lymph nodal disease. It should be remembered that many histological types do not concentrate on FDG and FDG also shows false-positive results in many other conditions like infection-inflammation; however, at the same time, it delivers high negative predictive values, an important consideration when employing FDG-PET/CT in the CUP scenario. SSTR-based PET/CT plays a pivotal role in primary diagnosis, staging, therapy planning, and response assessment in CUPs with neuroendocrine tumor or neuroendocrine neoplasm histology. The last two decades has witnessed great advancement in PET instrumentation and radiopharmaceuticals: particularly techniques like PET/magnetic resonance imaging and radiopharmaceuticals like FAPI (fibroblast-activation protein inhibitor)-based PET tracers. Hence, the role of PET/CT is expected to expand its reach in the coming years in line with accruing literature evidence, thereby upgrading its role and reliability in oncological practice strategies.
Keywords
USG - PET - PET-CT - FDG - FNAC - biopsy - carcinoma of unknown primary (CUP)Publication History
Article published online:
01 March 2023
© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Most of the established guidelines mention and recommend the use of FDG-PET/CT (fluorodeoxyglucose positron emission tomography/computed tomography) in carcinoma of unknown primary (CUP) especially in head–neck squamous cell carcinoma; as described in this article, this forms a powerful one-stop shop in diagnosing and staging modality and has multiple applications in difficult situations of CUPs. Although not used as a screening modality, FDG-PET/CT is recommended as the primary imaging modality in the evaluation of primary, staging, and response evaluation for CUP with histology known to demonstrate FDG avidity, especially patients presenting with lymph nodal disease. It should be remembered that many histological types do not concentrate on FDG and FDG also shows false-positive results in many other conditions like infection-inflammation; however, at the same time, it delivers high negative predictive values, an important consideration when employing FDG-PET/CT in the CUP scenario. SSTR-based PET/CT plays a pivotal role in primary diagnosis, staging, therapy planning, and response assessment in CUPs with neuroendocrine tumor or neuroendocrine neoplasm histology. The last two decades has witnessed great advancement in PET instrumentation and radiopharmaceuticals: particularly techniques like PET/magnetic resonance imaging and radiopharmaceuticals like FAPI (fibroblast-activation protein inhibitor)-based PET tracers. Hence, the role of PET/CT is expected to expand its reach in the coming years in line with accruing literature evidence, thereby upgrading its role and reliability in oncological practice strategies.
Introduction
Carcinoma of unknown primary (CUP) is defined as histology-proven malignancy whose organ of origin remains unidentified even after thorough investigations. This thoroughness and pre-work-up investigations are not fully defined, in fact it is a dynamic concept, expanding its scope and depth with newer developments including diagnostic studies. Newer advances in understanding molecular make-up of various malignancies, such as immunohistochemistry (IHC), and its clinical translation have revolutionized oncology practice in the last two decades. Likewise, positron emission tomography/computed tomography (PET/CT) hybrid imaging is a powerful modality that examines molecular processes/receptors in hand with an anatomical picture in a single go. Such a noninvasive single test that images the whole body with added molecular information becomes an attractive option in the CUP sitting. The role of PET/CT in the present day is based on available data and recommendations are summarized in this short review.
Risk Factors and Etiopathogenesis
Oncogenic mutations and environmental factor accepted for precipitating other known malignancies are identified as risk factors in CUP also, e.g., heavy smoking, central obesity, and alcohol consumption have been reported to have associations with CUPs.[1] Human papilloma virus subtypes are found to be associated with few forms of head–neck squamous cell carcinoma (SCC)-CUPs.[2] However, an association of germline mutations is not well established, and based on current literature the mutations are thought not related to genetic predisposition.[3] As per the Spanish Pathology Society consensus statement, angiogenesis activation (50–89%), oncogene over-[removed]10–30%), hypoxia-related proteins and epithelial–mesenchymal transition markers (16–25%), and the activation of intracellular signals (e.g., MAPK) are identified as vital drivers in the pathogenesis of CUPs.[4]
Epidemiology and Clinical Presentation: India and Global
The incidence of CUP worldwide during the last decade was 3 to 6 cases per 10,000,00 population in different series. As per National Cancer Registry Program, the figure reflects the same for Indian population also.[5] Overall, CUPs contribute to 2 to 3%-of all cancer cases.[6] A recent analysis of population-based registries showed that the incidence rate of CUP has been decreasing over time (from its peak around the 1990s to the present era). The reasons for this decline may be related to improvements in diagnostic methods such as advanced imaging and molecular profiling.[7]
Commonly presenting clinical complaints of CUP depend on the area involved and type of primary such as squamous cell, adenocarcinoma, poorly differentiating carcinoma, etc. Adenocarcinoma is the most prevalent pathological subtype reported in the CUP setting, followed by poorly differentiated cancers, SCCs, and neuroendocrine tumors (NETs).[8]
Overview of Currently Published Imaging Referral Guidelines (NCCN/ASCO/ESMO/RCR/IRIA/NCG/EANM): Where Do CECT, MRI, and PET-CT Stand?
The most updated National Comprehensive Cancer Network (NCCN) guidelines (version 1.2022) states that PET/CT imaging has added benefits to CT or PET alone. This modality has intermediate specificity and high sensitivity in a few small studies, larger studies are warranted to determine the clinical utility and role of PET/CT compared with conventional imaging modalities in CUPs. It is not used routinely in the initial evaluation of suspected metastatic malignancy and warranted in a few special conditions like while consideration of local/regional therapy and curative intent treatment is being planned in a suspected single site. The NCCN guideline also mentions PET/magnetic resonance imaging (MRI) as an alternative to PET/CT and its added benefits like lower doses of ionizing radiations with similar or even improved accuracy.
Current ASCO (American Society of Clinical Oncology) statement (version: 2020) on “Squamous Cell Carcinoma of Unknown Primary in the Head and Neck” states that along with meticulous clinical history and examination, contrast-enhanced CT (CECT) is the first-line modality, and this will identify the majority of primary tumors. When a primary is not evident on clinical examination and CECT, PET/CT should be the next diagnostic step yet before any endoscopic biopsies if the primary remains unknown. It has added utility especially in directing biopsy, palatine tonsillectomy, or lingual tonsillectomy. This permits the dual advantage of guiding biopsies and reducing the false-positive rate.[9]
The European Society for Medical Oncology (ESMO) 2015 guidelines suggests the use of FDG-PET/CT (fluorodeoxyglucose positron emission tomography/computed tomography) for diagnosis and staging of CUP tumors and especially those with cervical adenopathies and single metastasis. But for other CUPs, the role of FDG-PET is limited and this imaging procedure is not mandatory in the systematic work-up.[10] Similarly, the Royal College of Radiology (RCR) in their recommendations (2014) mentions using FDG PET/CT in locating a primary tumor in metastatic SCC of the neck, while also mentions about variable accuracy for this modality of CUP at other places.[11]
As per the published guidelines and recommendations in oncologic imaging of Indian Radiological and Imaging Association (IRIA/ICRI) (April 2020), PET/CT and MRI of the neck should be requested (before biopsy of high-risk sites) as the first line. PET/CT is also of value in assessing the presence of recurrent disease. In patients with dysphagia having a known postcricoid or upper esophageal disease, PET/CT scan is recommended. Where there is a high clinical index of suspicion of recurrence, it would be sensible to perform MRI or CT to assess the extent of the recurrence. In cases where there is clinical doubt, or where recurrence is under evaluation, it may be more appropriate to proceed directly to PET-CT.[12]
As per the published treatment protocol of NCG (National Cancer Grid) India (April 2017), cervical nodes when evaluated with fine needle aspiration cytology (FNAC) first turns out to be SCC of head and neck (SCCHN) or adenocarcinoma of unknown origin, and FDG-PET/CT can form one of the vital initial recommended investigations along with tumor markers and IHC. This would help guide and track the primary and also plan therapy adequately (decision making between neck dissection, adjuvant radiotherapy, concurrent chemotherapy, and so on). PET-CT is recommended as the first investigation in the clinical setting of recurrent SCCHN which is grossly defined as locoregional or distant metastatic and guides further treatment algorithm depending on disease extent and resectability. As per consensus statement of the Spanish Society of Pathology and the Spanish Society of Medical Oncology (2018), 60%-of poorly differentiated malignancies eventually turn out to be lymphomas, and in such cases, when present with widespread lymphadenopathies and having liver or spleen involvement, it is advisable to analyze along with FDG-PET/CT scan and repeat biopsies to guide the use of specific therapies.[4] The American College of Radiology appropriate use criteria statement has currently listed this entity under topics that are currently “under evaluation.” The EANM (European Association of Nuclear Medicine) procedural guidelines in tumor imaging have mentioned “searching for an unknown primary tumor when metastatic disease identified or when the patient presents with a paraneoplastic syndrome” and it forms an important indication of FDG-PET/CT.[13] The Society of Nuclear Medicine and Molecular Imaging (SNMMI) in their appropriate use criteria statement or somatostatin receptor (SSTR) PET for NETs states that the use of PET is appropriate (and considered acceptable) in “localization of primary tumor in patients with known metastatic disease but unknown primary with NET.”[14]
Clinical and Diagnostic Work-Up in CUP (Excluding Imaging)
The preliminary clinical work-up consists of a detailed history with particular attention to lifestyle, addictions, environmental factors, and previous treatments received. This is followed by extensive physical examination, including oropharyngeal, genitourinary, rectal examinations, and breast examinations in females. After the clinical evaluation, the important laboratory tests like complete blood counts with peripheral smear, urine analysis, liver and renal function tests, stool examination, and tumor markers such as serum PSA, serum CEA, serum AFP, serum Ca-125, etc., histological examinations, FNACs, and biopsy, are diagnostic choice and play an important role in the classification of tumor and guide further diagnostic procedures and therapies. IHC (e.g., S-100, TTF-1, cytokeratin, chromogranin, etc.) helps to delineate tumor lineage.
Imaging Guidelines
Screening
FDG-PET/CT has a high incidence of false-positive findings (mainly benign inflammatory lesions which are amongst the primary differentials in the CUP setting) and also false-negative findings (non-FDG-concentrating tumors such as well-differentiated NETs). Certain shortcomings of FDG-PET/CT as a screening investigation are noteworthy, viz. large amounts of image data and complex and time-consuming interpretation procedure; possibility of overlooking subtle pathological findings or over-diagnosis increasing the number of negative biopsies.[15] It also exposes an individual to high radiation doses (up to 25 mSv per study). Thus, it is recommended to use nonradioactive and targeted tests such as tumor markers before posting patients for PET/CT. In summary, FDG-PET/CT is not warranted for screening modality in suspected CUPs.
Diagnosis of Primary Malignancy in CUP
Functional or metabolic changes on PET-CT can occur in the absence of any corresponding anatomical changes and/or not visualized by anatomical imaging. A meta-analysis investigated the diagnostic performance of FDG-PET/CT and reported a primary tumor detection rate of 37%.[16] The role of FDG-PET/CT in head–neck SCCs and poorly differentiating cancers turning out to be lymphomas is well established in various guidelines as outlined above. In a subset of six reviewed studies, FDG-PET led to a change in treatment in 24.7%-of cases. PET-CT had the highest accuracy for detection of primaries in the hypopharynx and larynx.[17] [18] For the extra-cervical metastases, there is lack of a thorough assessment of diagnostic performance with heterogeneous results, and the modality appears comparable to other modalities. Among the various sites, the lung, pharyngeal, and pancreatic cancers represent the most frequently detected primary tumors.
The oropharynx and the lung are the two most common locations of false-positive FDG-PET/CT results.[16] Frequently, metastatic lung lesions/nodules are not FDG avid, especially primary malignancies like thyroid, hepatobiliary tree, renal, and testicular cancers, and commonly show lung metastases that have low-grade or indeterminate FDG uptake. There are also multiple physiological variants, benign tumors, and inflammatory and infective diseases known to cause increased FDG uptake and mimic malignant disease.[19] At present, there is no guideline or systemic analysis/study that has demonstrated definitive clinical benefit of PET/CT over CECT in the primary diagnosis of lung and brain metastases of unknown origin.[19]
Similarly for skeletal lesions, common originating sites for metastases are primaries from the lung, prostate, gastric carcinoma, hepatobiliary tree, and kidney cancers, while for brain lesions, the commonly detected primaries are from the breast and lung. For these malignancies, FDG-PET/CT has lesser or comparable accuracy compared to CECT or targeted modalities like mammography. The role of FDG-PET/CT has been studied in a few retrospective studies with a reported sensitivity up to 60%, while no direct comparison with other modalities is yet available to investigate its benefit.[20] On failure of other imagining modalities such as CECT or MRI, the FDG-PET/CT can be considered in these situations, especially if this is expected to impact further management.
As mentioned earlier, thorough etiological and clinical evaluation is a must in CUP diagnosis and before targeting them for PET/CT. The usual clinical presentations are local swelling, discomfort or pain in any body part commonly the abdomen, dyspnea, cough, bone pain, weakness, fatigue, poor appetite, weight loss, unusual bleeding or discharge, pyrexia of unknown origin, change in bowel-bladder habits, drenching night sweats, etc. Depending on the organ system involved, it may also present as ascites in abdominal/pelvic malignancies like ovarian cancer. More attention is to be given to local examination where symptoms appeared first, e.g., otolaryngo-rhino and oral cavity examination for cervical nodal disease, breast examination in axillary nodal disease, adequate prostate, breast, lung, renal, or thyroid primary evaluation for bone disease, and groin, genital, and rectal areas to be examined primarily in inguinal nodal disease. Most of the times this is followed by tissue diagnosis using FNAC/biopsy. It is a consensus that the site identified above diaphragm will have primary above diaphragm only but not true every time, especially for brain and supraclavicular lymph nodal lesions. Furthermore, the PET/CT imaging protocols are to be decided depending on these pathological and clinical factors. This is to target specific areas along with the whole body for rare distant sites, e.g., puffed cheek view in suspected oral cavity lesions in cervical nodal disease, for mediastinal nodal masses dedicated lung protocol where even scar-like nodules are suspicious, while liver lesions are to be imaged with multiphase contrast-enhanced study in the well-distended stomach and bowel loops (using both oral and rectal contrast if needed), it is advised to screen total body (head to toe) including nodal stations in limbs for suspected melanomatous lesions.
In a primary evaluation of the CUP, dual time-point PET imaging (i.e., PET imaging at routine 1 hour and after a standard delay) is recommended in a case-based setting to enhance its sensitivity and specificity (FDG is not tumor-specific) [Supplemental material]. Focal FDG uptake is indeterminate to differentiate malignancy versus inflammation versus physiological uptake. A gradually increasing trend of FDG uptake over time has been shown to be preponderant in malignant cells, and a decreasing or constant trend has been shown in inflammatory/infectious processes. This has proven to increase the accuracy of the FDG-PET/CT studies.[21]
Thus, FDG-PET/CECT is recommended as primary imaging investigation to identify the primary site in CUPs, particularly those presented as lymph nodal disease ([Figs. 1] and [2]).
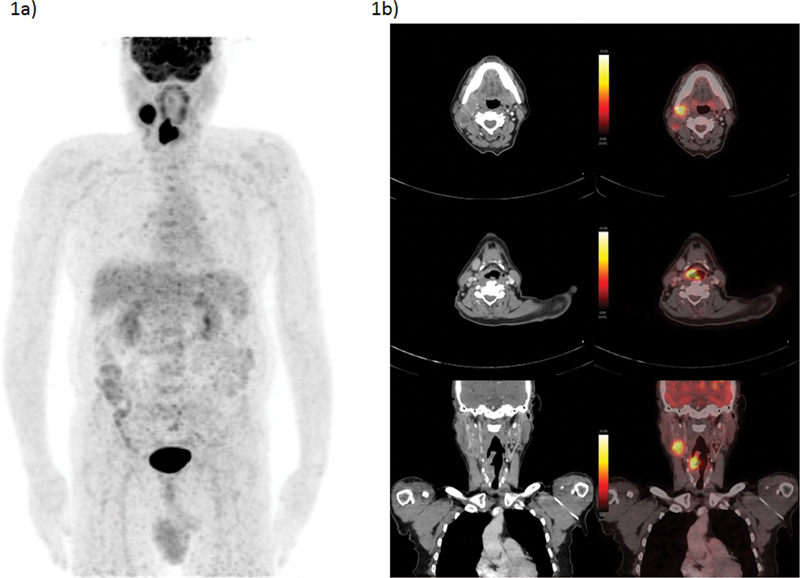
| Figure 1:A 62-year-old male presented with swelling over right-side neck over the last 1.5 months and cough of 20 days' duration. FNAC taken from neck mass turned out to be metastatic squamous cell carcinoma. He underwent FDG-PET/CT as baseline evaluation, which showed right-sided cervical lymph node, a few similar but smaller right level III nodes, and soft tissue lesion involving supraglottic larynx, arising from right-sided right PFS; no cartilage/hyoid erosion or extra-laryngeal involvement was noted. Direct laryngoscopy showed soft tissue growth involving the medial wall of right pyriform sinus. Biopsy taken from right-sided aryepiglottic fold showed squamous cell carcinoma. Considering no obvious evidence of distant metastases on present PET/CT scan, he was planned for curative surgical procedure. (a) FDG-PET/CT MIP image and (b) CECT and fused PET/CT transaxial and coronal section images showing FDG avid right-sided level II cervical node and FDG avid soft tissue lesion involving supraglottic larynx, arising from right PFS. The present illustration demonstrates the utility of FDG-PET/CT in both identifying primary and staging of nodal disease which impacts disease management, in this case being considered for curative locoregional approach. CECT, contrast-enhanced computed tomography; FDG-PET/CT, fluorodeoxyglucose positron emission tomography/computed tomography; FNAC, fine needle aspiration cytology; MIP, maximum intensity projection.
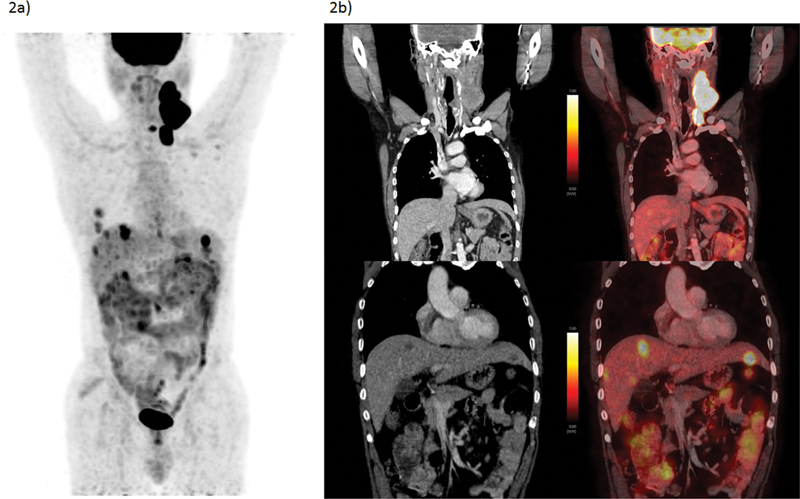
| Figure 2:A 59-year-old male presented with swelling over left-side neck over 2 months along with some dysphasia. FNAC taken from neck mass (lymph node) turned out to be metastatic squamous cell carcinoma. He also underwent direct laryngoscopy where punch biopsy from cricoid region came inconclusive. No other obvious lesion was identified. He underwent FDG-PET/CT, which showed metabolically active multiple left level II, III, IV nodes and nodal masses, left infraclavicular nodes, and liver lesions. USG-guided biopsy from the lesion was also identified as of similar histology (SCC) and a diagnosis of metastatic SCC-CUP was made. (a) FDG-PET/CT MIP image and (b) CECT and fused PET/CT coronal section images showing FDG left cervical nodal masses and metastatic FDG avid hypodense hepatic lesions. The present illustration demonstrates the inability to identify primary lesion and diagnosis of CUP in spite of multiple diagnostic approaches as well as the utility of FDG PET/CT staging where unusually site for SCC such as liver was identified. CECT, contrast-enhanced computed tomography; CUP, carcinoma of unknown primary; FDG-PET/CT, fluorodeoxyglucose positron emission tomography/computed tomography; FNAC, fine needle aspiration cytology; MIP, maximum intensity projection; SCC, squamous cell carcinoma; USG, ultrasonography.
FDG-PET/CT is not recommended as the primary imaging investigation for CUPs presenting with pulmonary, bone, or brain metastases and also not the primary imaging investigation with histological subtypes with indeterminate FDG concentration (e.g., mucinous carcinoma or serous adenocarcinoma). However, it may be considered when conventional imaging modalities such as CECT and extensive clinical work-up have failed to find primary and also in treatment response monitoring scenarios ([Figs. 3] and [4]).
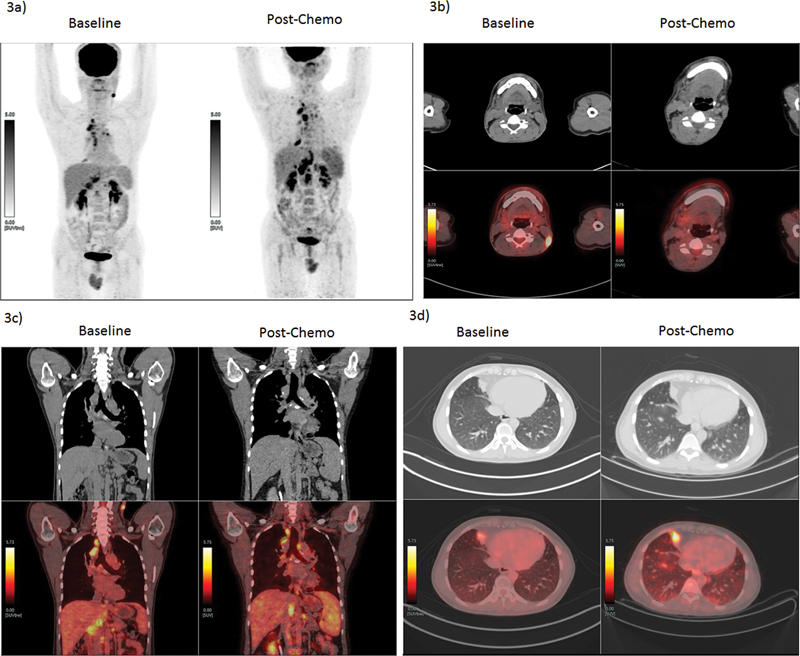
| Figure 3:A 33-year-old male, occasional smoker, presented with left-sided neck swelling. The biopsy from left level III lymph nodes turned out to be metastatic deposits of squamous carcinoma. He underwent 18F-FDG PET/CT to find primary and baseline staging. The FDG-PET/CT revealed metabolically active left-sided neck node (level V), multiple mediastinal nodes, right middle lobe paracardiac lesion, and abdominal nodes. Biopsy was attempted from chest lesion and mediastinal nodules, which revealed similar histology and other infective etiologies were ruled out. A diagnosis of metastatic SCC-CUP was made. The patient further received palliative chemotherapy as per protocol (paclitaxel and carboplatin). The response evaluation using FDG PET/CT showed response to cervical lesion but there was an increase in size and metabolism of other lesions, with multiple new lung nodules noted. The present therapy was stopped and molecular analysis was underway for the patient to decide further therapeutic options. (a) PET MIP images pre- and post-chemotherapy showing disease progression, (b) CT and PET/CT fused transverse section images pre- and post-chemotherapy at level V node showing complete resolution of the lesion. (c) CT and PET/CT fused coronal section images pre- and post-chemotherapy showing disease progression in abdominal and mediastinal nodes. (d) CT and PET/CT transverse section images pre- and post-chemotherapy showing progression of pleural-based nodule and newly seen tiny pulmonary nodules. The present illustration demonstrates the utility of FDG PET/CT in staging disease in CUP and response evaluation to systemic chemotherapy. CUP, carcinoma of unknown primary; 18F-FDG-PET/CT, fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography; MIP, maximum intensity projection; SCC, squamous cell carcinoma.

| Figure 4:A 62-year-old male presented with right hip and lower back pain for 3 months, MRI spine showed extensive marrow replacing lesion in the pelvic bones and vertebrae suggestive of metastatic deposits. FDG-PET/CT was considered to look for primary lesion and characterization of skeletal lesions, which showed FDG avid left lung mass which was suspected for primary lesion along with other tiny nodules. FDG avid metastatic appearing mediastinal, supraclavicular, and abdominal nodes were noted along with FDG avid lesions in the brain, liver, spleen, left kidney, right adrenal, and skeletal system (including multiple vertebrae, pelvis, sacrum, and bilateral femur). The USG-guided biopsy from left supra-clavicular node showed poorly differentiated non-small cell carcinoma. The disease was diagnosed as metastatic lung cancer with multiple soft tissue (including brain) and skeletal metastatic lesions. (a) FDG-PET/CT MIP images showing multiple regions of pathological skeletal and multiple other lesions scattered over the whole body. (b) CECT and PET/CT fused transverse section images, showing FDG concentrating left lung upper lobe mass and mediastinal nodes, enhancing lesion in right temporal lobe, and sagittal section image showing sclerotic lesions in multiple vertebrae and pelvic bones. The present illustration demonstrates the utility of FDG-PET/CT in guiding primary diagnosis and whole-body disease staging in evaluation of a patient presenting with metastatic skeletal disease. CECT, contrast-enhanced computed tomography; FDG-PET/CT, fluorodeoxyglucose positron emission tomography/computed tomography; MIP, maximum intensity projection; MRI, magnetic resonance imaging; USG, ultrasonography.
FDG-PET/CT-Guided Biopsy in CUP
FDG-PET can differentiate between the metabolically active viable portions of the tumor from the nonactive part of the tumor; biopsy from the area of highest metabolic activity is expected to result in greater diagnostic yield, especially in suspected abdominal soft tissue, bone, and head–neck primaries. This approach and quantification method helped verify biopsy adequacy through a comparison of specimen activity and that expected from the PET image.[22] Yet, its exact utility in CUPs and direct comparisons with a CT-guided approach are not established, thus it is recommended primarily for FDG-concentrating lesions on scan and with previously failed biopsy sampling ([Flow Chart 1]).
SSTR PET/CECT in Biopsy-Proven NEN in Unknown Primary (CUP-NEN)
CUP syndrome is common in metastatic neuroendocrine neoplasms (NENs), and in recent years SSTR-based PET-CT has emerged as a strong imaging tool with the highest accuracy. As per the above-mentioned guidelines, SSTR-based PET/CT is recommended in low-grade NETs to search primary lesions with greater accuracy. A study by Sampathirao et al used combined dual-tracer PET/CT (both 68Ga-DOTA-TATE/NOC and FDG) correlating with MIBI-1/Ki-67 index in 51 patients of metastatic CUP-NETs and reported a sensitivity of 60.78%-for the former, with an overall lesion detection sensitivity of 96.87%.[23] Small-sized pancreatic and ileum/jejunal primaries are most commonly encountered, which are missed in previous investigations. Absent SSTR expression such as in de-differentiated or high-grade tumors results in false-negative results,[23] where FDG becomes the tracer of choice. Thus, SSTR PET/CECT (68Ga-DOTA-TATE/NOC) is recommended as a primary imaging modality in biopsy-proven NEN in unknown primary, especially for the grade 1 and 2 tumors ([Figs. 5] and [6]).

| Figure 5:A 60-year-old female presented with right upper abdomen discomfort and with USG suggestive of multiple mass lesions in the liver; patient got evaluated further with CECT scan and liver lesions were labelled not typical for HCC though they showed arterial enhancement with abdominal nodes. Her serum chromogranin A level was highly raised; thus, she was referred for 68Ga-DOTATATE PET/CT scan to ascertain the primary and also evaluate disease extent. 68Ga-DOTATATE PET/CT revealed SSTR-expressing mesenteric mass, abdominal nodes, liver lesions, and incidentally detected well-defined left lateral ventricular lesion in the brain, for which the patient was asymptomatic. A biopsy from the liver lesion revealed this to be grade I (Mib-1 index of 1%) neuroendocrine tumor. The disease was labelled as metastatic mesenteric NET with nodal and liver metastases and incidentally detected meningioma/? cerebral lesion. The patient was started on long-acting octreotide injections; she revealed stable and asymptomatic disease post 6 months. (a) 68Ga-DOTATATE PET/CT MIP image, showing SSTR-expressing mesenteric lesion, liver lesions (Krenning score 4), and left cerebral lesion. (b) CECT and PET/CT fused transaxial section images showing SSTR-expressing mesenteric lesion, liver lesions, and left cerebral (ventricular) lesion. The present illustration demonstrates the utility of SSTR-based PET/CT in guiding primary diagnosis, disease staging-grading, and guiding therapy in evaluation of NETs presenting with metastatic disease. CECT, contrast-enhanced computed tomography; HCC, hepatocellular carcinoma; NET, neuroendocrine tumor; PET/CT, positron emission tomography/computed tomography; SSTR, somatostatin receptor; USG, ultrasonography.
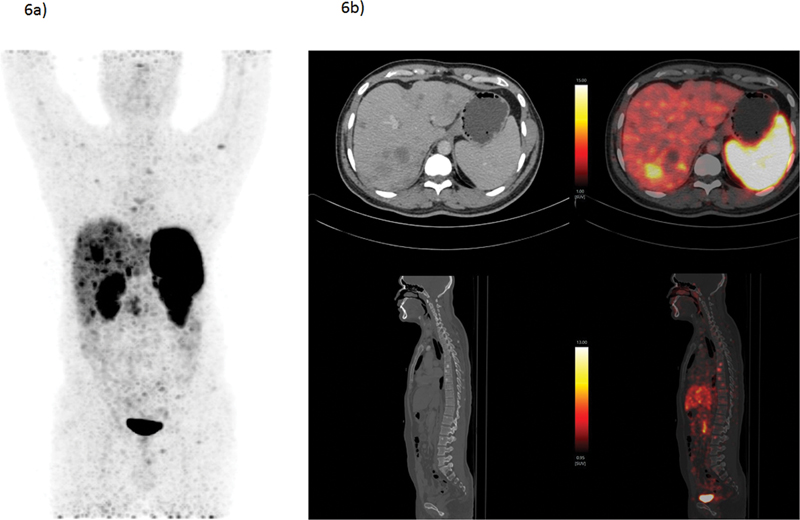
| Figure 6:A 34-year-old male presented with generalized weakness and abdominal discomfort, showed multiple liver lesions and splenomegaly on CECT. The liver biopsy revealed to be grade-II NET with Mib-1 index of 13%. He underwent baseline 68Ga-DOTATATE PET/CT scan to find primary and also disease extent. 68Ga-DOTATATE PET/CT revealed SSTR-expressing multiple bilobar liver lesions and sclerotic skeletal lesions; however, no obvious primary lesion could be found. The patient was diagnosed as grade II metastatic NET with unknown primary and being evaluated for Chemo-PRRT treatment protocol considering SSTR expressing (Krenning score 3) multiple liver and bone lesions. (a) 68Ga-DOTATATE PET/CT MIP image showing SSTR-expressing bilobar liver lesions (Kenning score 3) and multiple skeletal lesions. (b) CECT and PET/CT fused transverse section images showing SSTR-expressing liver lesions; sagittal section showing multiple sclerotic skeletal lesions. The present illustration demonstrates the utility of SSTR-based PET/CT in guiding diagnosis and disease staging in evaluation of CUP-NETs presenting with metastatic disease. CECT, contrast-enhanced computed tomography; MIP, maximum intensity projection; NET, neuroendocrine tumor; PET/CT, positron emission tomography/computed tomography; SSTR, somatostatin receptor;
Staging
FDG-PET/CT, due to its high sensitivity and whole-body imaging ability, offers advantages to identify or rule out additional metastatic sites, which may have important significance for patient management. This is highlighted in multiple current practice guidelines for patients with CUP who present with only lymph node metastatic disease. Many times, these patients are being planned for radical or definitive treatments such as surgery or radiation therapy (RT) in restricted local disease. Here exact disease staging is warranted, which has important therapeutic and prognostic consequences. FDG-PET/CT performs better than most other modalities and serves as a one-stop-shop modality mapping disease in the whole body in a single examination. It aids in the decision-making investigation, as mentioned in the above guidelines, from the viewpoint of whether to plan for definitive therapy or adjuvant palliative therapies. It is also further advised to get a pathological diagnosis when the findings change the management drastically, considering the false-positive rates.[24] A systematic review in cervical nodal diseases of 16 studies on 302 patients stated that FDG-PET detected previously unrecognized metastases in 27%-of patients, of which 11%-were distant metastases, and resulted in changes in treatment in 24.7%-of cases.[17] Hence, FDG-PET/CT is recommended as the primary staging modality in CUPs with histological subtype known to be FDG concentrating or avid (e.g., SCC, poorly differentiated) ( [Figs. 1],[2],[3] ).
The SSTR-based PET/CT delineates the primary tumor site and frequently demonstrates additional metastatic lesions which were not previously diagnosed on ultrasonography (USG)/CT considering their indolent nature. This provides better staging that results in clinically relevant changes in management in about one-third of patients.[25] Although finding more lesions is not prognostically important in the NET setup, it provides a baseline scan and aids in therapy decision-making and assessment of therapeutic response like peptide receptor radionuclide therapy (PRRT) or chemotherapy. Furthermore, the concept of dual-tracer PET is now well established in NENs and fast become an important gatekeeper for therapeutic disease making amongst the different therapy options. In summary, SSTR-based PET/CT is recommended as the primary staging modality in CUPs with NEN histology.
Management and Imaging Response Assessment in Neoadjuvant, Adjuvant, and Palliative Settings
Presently treatment strategies have shifted from empirical cytotoxic therapies to identifying the primary tumors and targeted therapies as per the tumor type.[26] Even though the source of primary is not identified, the limited disease is subjected to curative interventions such as surgical excision or RT mainly in head–neck lymph nodal disease. Conventional anatomical agents provide lower accuracy considering posttreatment dysmorphic normal anatomy and disruption of normal tissue planes. Multiple groups and studies have emphasized FDG-PET/CT as a modality of choice in response monitoring in head and neck cancers.[27] [28] Further standardized evaluation criteria have been developed in this group, such as Hopkins[29] and PERCIST[30] are based upon PET-CT and greatly influencing response evaluation in oncology. Although there is no direct comparison available in CUPs between other modalities and PET, it is imperative that PET/CT provides more confidence to clinicians for labeling patients disease-free owing to its high negative predictive value. In conclusion, FDG-PET/CT is recommended as a first-line response evaluation modality for known FDG-concentrating histology subtype ( [Fig. 3] ).
Neoadjuvant systemic therapy option has been followed in clinical practice to deliver the best possible outcome in a number of malignancies and FDG-PET/CT has demonstrated its added role and higher confidence in neoadjuvant management of esophageal, rectal,[31] and breast cancers, a similar way it is thought to aid in CUP management when baseline disease is FDG-concentrating. As per recent NCCN guideline (1.2022), when CUPs are diagnosed as disseminated disease, which is the scenario in many encountered cases, systemic therapy with cytotoxic drugs or immune-modulatory therapy even sometimes combined with RT is practiced. As per one study on head neck cancers,[32] the accuracy of PET in therapy response assessment was significantly better than conventional imaging (CT/MRI) (p < 0.001). Early response evaluation with FDG-PET provides insights into functional characteristics before anatomical changes, which provides lead time. It is now established that anatomical response evaluation criteria based upon tumor volume cannot quantify nonmalignant or the fibrous material adequately in the tumor.[33] In addition, the guidelines also mention immunomodulatory therapies and trials where response patterns differ from those of chemotherapeutic and molecularly targeted agents considering the recruitment of immune cells and related changes in the tumor microenvironment. This has been highlighted in recently approved SNMMI procedure standards[34] and introduces new terms like pseudo-response, hyper-response, dissociated-response, and durable response. FDG-PET additionally has shown accuracy in monitoring systemic immune response and detecting immune-related adverse effects in the early stages.[35] [36] Overall, FDG-PET/CT response is better correlated with patient outcome and considered a better predictor for the effectiveness of new anticancer therapies than routine anatomical criteria ([Flow Chart 2]).
In summary, FDG PET/CECT is recommended as a response evaluation modality for FDG-concentrating tumor types, following systemic therapies either in neoadjuvant, adjuvant, or palliative therapy setting (cytotoxic chemotherapies, immune-modulatory therapy, other specialized approaches with or without concurrent radiation).
As per the appropriate use criteria statement by SNMMI[14] for SSTR-based PET/CT in NET, a well-established practice in both pretherapy patient classification and posttherapy patient monitoring, the complete anatomical response is observed in <10 href="https://www.thieme-connect.com/products/ejournals/html/10.1055/s-0042-1760311#FI221190798-9" xss=removed>Flow Chart 3]).[37]
Thus, SSTR-based PET/CT is recommended for response evaluation in CUPs with NET histology who are on systemic therapies (octreotide, PRRT, chemo-PRRT).
Follow-Up
The follow-up frequency and strategy in CUP is done differently considering the vast spectrum of diseases and outcomes. Routine follow-up especially in asymptomatic patients would not justify for unnecessary ionizing-radiation exposure in the form of FDG-PET/CT. Also, many false-positive findings in common benign conditions can lead to over-diagnosis. However, FDG PET/CT is thought to be justified in previously known FDG-concentrating disease when routine investigations like tumor markers/FNAC/USG are inconclusive in symptomatic patients ([Flow Chart 4]).
In summary, FDG-PET/CT is not recommended as a routine follow-up imaging modality in the posttreatment scenario in CUPs. The modality can be considered in the follow-up of symptomatic patients with inconclusive investigations and previously FDG-concentrating disease.
Future Directions
PET/MR
MRI brings together excellent anatomical resolution with functional information such as diffusion-weighted imaging. These parameters have been reported to differentiate benign and malignant lesions[38] with substantial success. This functional information in hand with PET data is perceived to aid in primary tumor detectability more efficiently and allow characterization of lesions.[16] Furthermore, PET/MRI reduces radiation exposure to the patient to a great extent. In a comparative study between whole-body PET/CT and PET/MR, both modalities provided comparable diagnostic ability. In another study comparing contrast 3T multiparametric MRI with FDG-PET/CT in HN-SCC CUP patients, both modalities performed similarly, though PET/CT proved to be more sensitive while mp-MRI to be more specific, in such a way that a combined PET/MR approach is expected to deliver the greatest accuracy. Patients with pelvic lymphadenopathy or skeletal lesions suspicious for primary prostate malignancy especially with raised serum PSA levels can be imaged with 68Ga-PSMA-11 PET/MR with high imaging sensitivity and specificity. Such PSMA PET-assisted robotic trans-gluteal biopsy also has been reported in view of its high diagnostic yield and better safety profile, especially with prior negative results at transrectal USG-guided biopsy.[39] With growing experience in PET/MR techniques, this modality appears to deliver powerful output in CUP scenarios.
Newer PET Tracers: 68Ga-FAPI-Based PET-CT in CUP
A tracer that targets the fibroblast activation protein factor (universally present in tumor stroma) has been of immense interest recently in oncology after its initial experiences in a spectrum of cancers.[40] It has shown multiple benefits over FDG, such as nonrequirement of fasting status and better physiological bio-distribution (e.g., no uptake in the brain and gastrointestinal tract as FDG). In its initial experiences in head–neck CUPs, fibroblast-activation protein inhibitor (FAPI)-PET/CT could additionally detect the primary tumor in 7 out of 18 patients (38.89%), which were negative in FDG-PET/CT.[41] With gratifying results in multiple solid tumors (most gastrointestinal, genitourinary cancers) which are indeterminate on FDG, FAPI is postulated to be a potential player in CUP management. According to the present limited expertise and literature, it is not used as a direct replacement for FDG in CUP scenarios; whenever available, FAPI-PET can be tried post-negative-FDG PET/CT results or in selective indications where histopathology is suspicious for peritoneal/gastric signet ring cell serous carcinoma and no primary lesion could be found on any previous examination.
Similarly, 68Ga-Pentixafor PET/CT that targets CXCR4 (chemokine receptor-4) over-expressed in multiple hematological malignancies including multiple myeloma and solid tumors where FDG underperforms. However, its exact utility in the CUP scenario is yet to be established.
alient Points
FDG-PET/CT is not to be used for screening modality.
FDG-PET/CT is recommended as the primary imaging modality in evaluation of primary, staging, and response evaluation for CUP histology known to have FDG concentration, especially CUPs presenting as lymph nodal disease.
The exact choice of imaging protocol strongly depends on the commonly suspected sites as per symptomatology and clinical findings for every single case. Dual time-point imaging should be routinely used in the labelling of indeterminate lesions with FDG concentration.
FDG PET/CT is not recommended as a primary imaging modality in CUPs presenting as bone/brain/lung/liver disease and histology known to have indeterminate FDG concentration. But FDG PET/CT can be considered with failure of other initial imaging and biopsies.
SSTR-based PET/CT is recommended as a primary imaging modality in evaluation primary, staging, therapy planning, and response evaluation of CUPs with NET histology type.
If available, FAPI PET/CT can be tried post-negative-FDG PET/CT or other noninvasive tests results, as well as in selective indications where histopathology is suspicious for peritoneal/gastric signet ring cell serous carcinoma and no primary lesion could be found on any previous examination.

| Flowchart 1:Screening, baseline diagnosis, and staging.
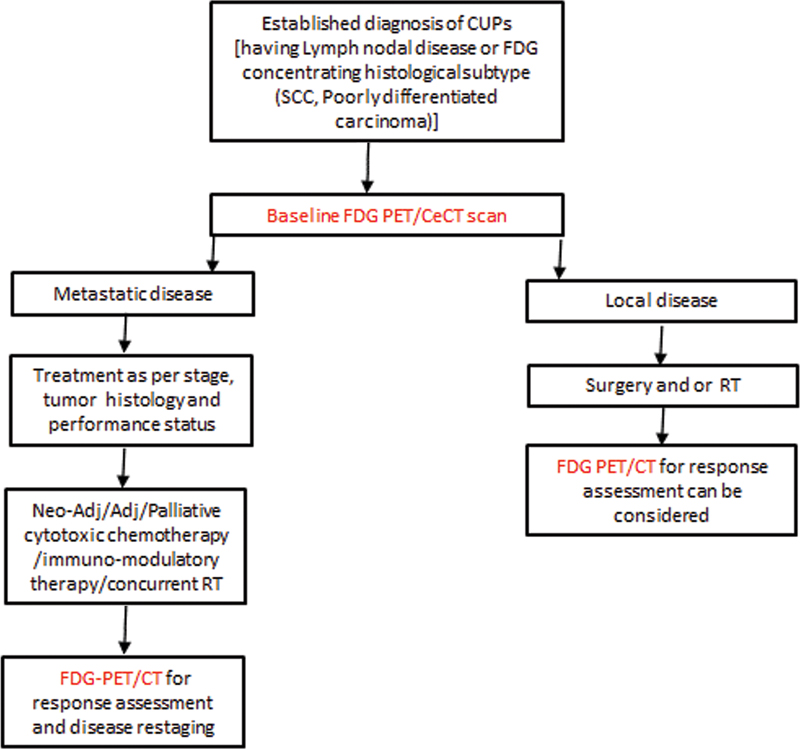
| Flowchart 2:Treatment response assessment in CUP with FDG-PET/CT.
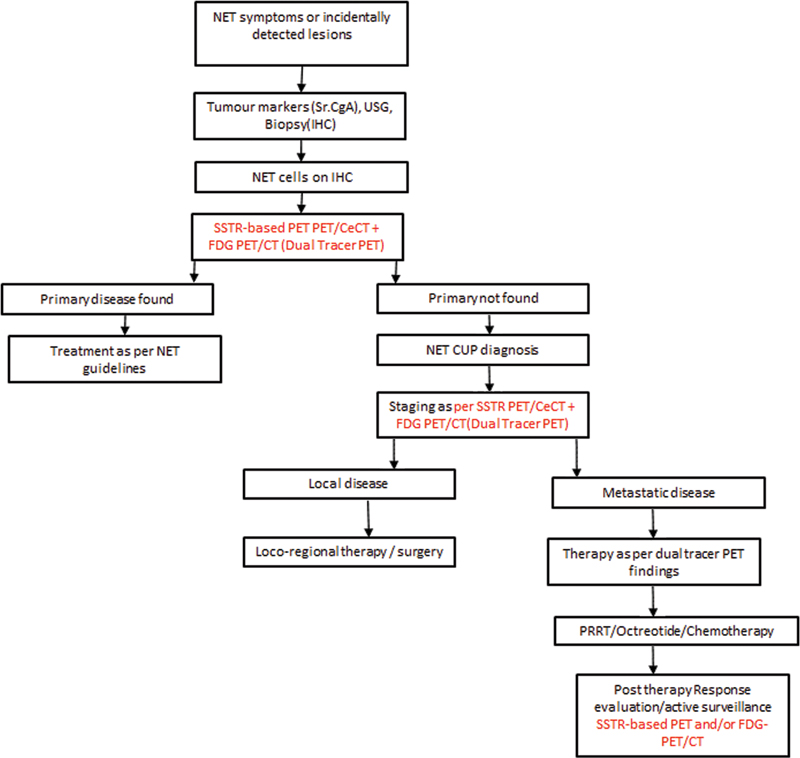
| Flowchart 3:An algorithmic CUP-NET management strategy.
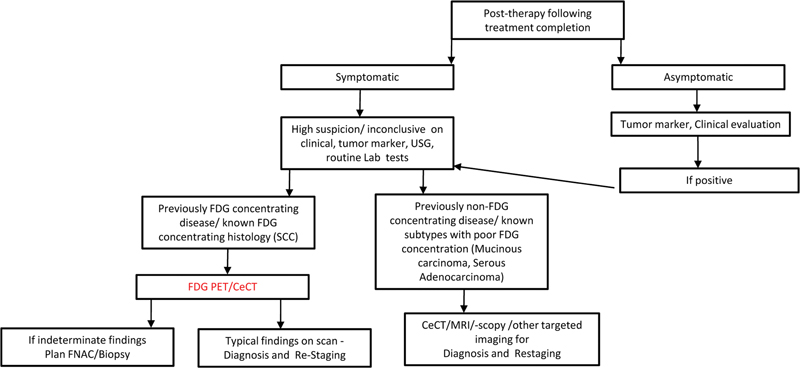
| Flowchart 4:Follow-up in CUP following completion of treatment.
Conflict of Interest
None declared.
Acknowledgements
The authors acknowledge the help of Dr. A. Nazaar for preparing the images at places.
Supplementary Material
References
- Varghese AM, Arora A, Capanu M. et al. Clinical and molecular characterization of patients with cancer of unknown primary in the modern era. Ann Oncol 2017; 28 (12) 3015-3021
- Rassy E, Nicolai P, Pavlidis N. Comprehensive management of HPV-related squamous cell carcinoma of the head and neck of unknown primary. Head Neck 2019; 41 (10) 3700-3711
- Hemminki K, Chen B, Kumar A. et al. Germline genetics of cancer of unknown primary (CUP) and its specific subtypes. Oncotarget 2016; 7 (16) 22140-22149
- Losa F, Iglesias L, Pané M. et al. 2018 consensus statement by the Spanish Society of Pathology and the Spanish Society of Medical Oncology on the diagnosis and treatment of cancer of unknown primary. Clin Transl Oncol 2018; 20 (11) 1361-1372
- Mathur P, Sathishkumar K, Chaturvedi M. et al; ICMR-NCDIR-NCRP Investigator Group. Cancer Statistics, 2020: Report From National Cancer Registry Programme, India. JCO Glob Oncol 2020; 6: 1063-1075
- Pavlidis N, Pentheroudakis G. Cancer of unknown primary site. Lancet 2012; 379 (9824): 1428-1435
- Rassy E, Pavlidis N. The currently declining incidence of cancer of unknown primary. Cancer Epidemiol 2019; 61: 139-141
- Qaseem A, Usman N, Jayaraj JS, Janapala RN, Kashif T. Cancer of unknown primary: a review on clinical guidelines in the development and targeted management of patients with the unknown primary site. Cureus 2019; 11 (09) e5552
- Maghami E, Ismaila N, Alvarez A. et al. Diagnosis and management of squamous cell carcinoma of unknown primary in the head and neck: ASCO guideline. J Clin Oncol 2020; 38 (22) 2570-2596
- Fizazi K, Greco FA, Pavlidis N, Daugaard G, Oien K, Pentheroudakis G. ESMO Guidelines Committee. Cancers of unknown primary site: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015; 26 (Suppl. 05) v133-v138
- Scarbrook A. Carcinoma of unknown origin. In: Nicolson T. ed. Recommendations of Cross Sectional Imaging in Cancer Management. London: Royal College of Radiologists; 2014
- Anbarasu A, Joshi V, Solanki R, Kumar J, Shah D. Head & Neck Imaging Sub Specialty Group Scanning Guidelines Ct Scan/Mr Imaging Version April; 2020. Accessed December 13, 2022 at: https://icri.iria.org.in/head-and-neck-imaging-guidelines-2020
- Boellaard R, Delgado-Bolton R, Oyen WJ. et al; European Association of Nuclear Medicine, (EANM). FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging 2015; 42 (02) 328-354
- Hope TA, Bergsland EK, Bozkurt MF. et al. Appropriate use criteria for somatostatin receptor PET imaging in neuroendocrine tumors. J Nucl Med 2018; 59 (01) 66-74
- Schöder H, Gönen M. Screening for cancer with PET and PET/CT: potential and limitations. J Nucl Med 2007; 48 (Suppl. 01) 4S-18S
- Kwee TC, Kwee RM. Combined FDG-PET/CT for the detection of unknown primary tumors: systematic review and meta-analysis. Eur Radiol 2009; 19 (03) 731-744
- Rusthoven KE, Koshy M, Paulino AC. The role of fluorodeoxyglucose positron emission tomography in cervical lymph node metastases from an unknown primary tumor. Cancer 2004; 101 (11) 2641-2649
- Lee JR, Kim JS, Roh JL. et al. Detection of occult primary tumors in patients with cervical metastases of unknown primary tumors: comparison of (18)F FDG PET/CT with contrast-enhanced CT or CT/MR imaging-prospective study. Radiology 2015; 274 (03) 764-771
- De Wever W, Meylaerts L, De Ceuninck L, Stroobants S, Verschakelen JA. Additional value of integrated PET-CT in the detection and characterization of lung metastases: correlation with CT alone and PET alone. Eur Radiol 2007; 17 (02) 467-473
- Park SB, Park JM, Moon SH. et al. Role of 18F-FDG PET/CT in patients without known primary malignancy with skeletal lesions suspicious for cancer metastasis. PLoS One 2018; 13 (05) e0196808
- Houshmand S, Salavati A, Segtnan EA, Grupe P, Høilund-Carlsen PF, Alavi A. Dual-time-point imaging and delayed-time-point fluorodeoxyglucose-PET/computed tomography imaging in various clinical settings. PET Clin 2016; 11 (01) 65-84
- Fei B, Schuster DM. PET molecular imaging-directed biopsy: a review. AJR Am J Roentgenol 2017; 209 (02) 255-269
- Sampathirao N, Basu S. MIB-1 index-stratified assessment of dual-tracer PET/CT with 68Ga-DOTATATE and 18F-FDG and multimodality anatomic imaging in metastatic neuroendocrine tumors of unknown primary in a PRRT workup setting. J Nucl Med Technol 2017; 45 (01) 34-41
- Fletcher JW, Djulbegovic B, Soares HP. et al. Recommendations on the use of 18F-FDG PET in oncology. J Nucl Med 2008; 49 (03) 480-508
- Barrio M, Czernin J, Fanti S. et al. The impact of somatostatin receptor-directed PET/CT on the management of patients with neuroendocrine tumor: a systematic review and meta-analysis. J Nucl Med 2017; 58 (05) 756-761
- Varadhachary, Gauri, James L.Abbruzzese. “Carcinoma of unknown primary.” Abeloff's Clinical oncology. Elsevier 2020; 1694-1702
- Gupta T, Master Z, Kannan S. et al. Diagnostic performance of post-treatment FDG PET or FDG PET/CT imaging in head and neck cancer: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging 2011; 38 (11) 2083-2095
- Slevin F, Subesinghe M, Ramasamy S, Sen M, Scarsbrook AF, Prestwich RJ. Assessment of outcomes with delayed (18)F-FDG PET-CT response assessment in head and neck squamous cell carcinoma. Br J Radiol 2015; 88 (1052): 20140592
- Marcus C, Ciarallo A, Tahari AK. et al. Head and neck PET/CT: therapy response interpretation criteria (Hopkins Criteria)-interreader reliability, accuracy, and survival outcomes. J Nucl Med 2014; 55 (09) 1411-1416
- Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med 2009; 50 (Suppl. 01) 122S-150S
- Cliffe H, Patel C, Prestwich R, Scarsbrook A. Radiotherapy response evaluation using FDG PET-CT-established and emerging applications. Br J Radiol 2017; 90 (1071): 20160764
- Martin RC, Fulham M, Shannon KF. et al. Accuracy of positron emission tomography in the evaluation of patients treated with chemoradiotherapy for mucosal head and neck cancer. Head Neck 2009; 31 (02) 244-250
- Evilevitch V, Weber WA, Tap WD. et al. Reduction of glucose metabolic activity is more accurate than change in size at predicting histopathologic response to neoadjuvant therapy in high-grade soft-tissue sarcomas. Clin Cancer Res 2008; 14 (03) 715-720
- Lopci E, Hicks RJ, Dimitrakopoulou-Strauss A. et al. Joint EANM/SNMMI/ANZSNM practice guidelines/procedure standards on recommended use of [18F]FDG PET/CT imaging during immunomodulatory treatments in patients with solid tumors version 1.0. Eur J Nucl Med Mol Imaging 2022; 49 (07) 2323-2341
- Ayati N, Sadeghi R, Kiamanesh Z, Lee ST, Zakavi SR, Scott AM. The value of 18F-FDG PET/CT for predicting or monitoring immunotherapy response in patients with metastatic melanoma: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging 2021; 48 (02) 428-448
- Wong ANM, McArthur GA, Hofman MS, Hicks RJ. The advantages and challenges of using FDG PET/CT for response assessment in melanoma in the era of targeted agents and immunotherapy. Eur J Nucl Med Mol Imaging 2017; 44 (Suppl. 01) 67-77
- Adnan A, Basu S. Discordance between histopathologic grading and dual-tracer PET/CT findings in metastatic NETs and outcome of 177Lu-DOTATATE PRRT: does in vivo molecular PET perform better from the viewpoint of prediction of tumor biology?. J Nucl Med Technol 2021; 50 (Suppl. 03) 248-255
- Ruhlmann V, Ruhlmann M, Bellendorf A. et al. Hybrid imaging for detection of carcinoma of unknown primary: a preliminary comparison trial of whole-body PET/MRI versus PET/CT. Eur J Radiol 2016; 85 (11) 1941-1947
- Kumar R, Singh SK, Mittal BR. et al. Safety and diagnostic yield of 68Ga prostate-specific membrane antigen PET/CT-guided robotic-assisted transgluteal prostatic biopsy. Radiology 2022; 303 (02) 392-398
- Kratochwil C, Flechsig P, Lindner T. et al. 68Ga-FAPI PET/CT: tracer uptake in 28 different kinds of cancer. J Nucl Med 2019; 60 (06) 801-805
- Gu B, Xu X, Zhang J. et al. The added value of 68Ga-FAPI PET/CT in patients with head and neck cancer of unknown primary with 18F-FDG-negative findings. J Nucl Med 2022; 63 (06) 875-881
- Niederkohr RD, Greenspan BS, Prior JO. et al. Reporting guidance for oncologic 18F-FDG PET/CT imaging. J Nucl Med 2013; 54 (05) 756-761
Address for correspondence
Sandip Basu, MBBS, DRM, DNB, MNAMSRadiation Medicine Centre, Bhabha Atomic Research Centre, Tata Memorial Hospital AnnexeJerbai Wadia Road, Parel, Mumbai, Maharashtra, 400 012 IndiaEmail: drsanb@yahoo.comPublication History
Article published online:
01 March 2023© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Figure 1:A 62-year-old male presented with swelling over right-side neck over the last 1.5 months and cough of 20 days' duration. FNAC taken from neck mass turned out to be metastatic squamous cell carcinoma. He underwent FDG-PET/CT as baseline evaluation, which showed right-sided cervical lymph node, a few similar but smaller right level III nodes, and soft tissue lesion involving supraglottic larynx, arising from right-sided right PFS; no cartilage/hyoid erosion or extra-laryngeal involvement was noted. Direct laryngoscopy showed soft tissue growth involving the medial wall of right pyriform sinus. Biopsy taken from right-sided aryepiglottic fold showed squamous cell carcinoma. Considering no obvious evidence of distant metastases on present PET/CT scan, he was planned for curative surgical procedure. (a) FDG-PET/CT MIP image and (b) CECT and fused PET/CT transaxial and coronal section images showing FDG avid right-sided level II cervical node and FDG avid soft tissue lesion involving supraglottic larynx, arising from right PFS. The present illustration demonstrates the utility of FDG-PET/CT in both identifying primary and staging of nodal disease which impacts disease management, in this case being considered for curative locoregional approach. CECT, contrast-enhanced computed tomography; FDG-PET/CT, fluorodeoxyglucose positron emission tomography/computed tomography; FNAC, fine needle aspiration cytology; MIP, maximum intensity projection.

| Figure 2:A 59-year-old male presented with swelling over left-side neck over 2 months along with some dysphasia. FNAC taken from neck mass (lymph node) turned out to be metastatic squamous cell carcinoma. He also underwent direct laryngoscopy where punch biopsy from cricoid region came inconclusive. No other obvious lesion was identified. He underwent FDG-PET/CT, which showed metabolically active multiple left level II, III, IV nodes and nodal masses, left infraclavicular nodes, and liver lesions. USG-guided biopsy from the lesion was also identified as of similar histology (SCC) and a diagnosis of metastatic SCC-CUP was made. (a) FDG-PET/CT MIP image and (b) CECT and fused PET/CT coronal section images showing FDG left cervical nodal masses and metastatic FDG avid hypodense hepatic lesions. The present illustration demonstrates the inability to identify primary lesion and diagnosis of CUP in spite of multiple diagnostic approaches as well as the utility of FDG PET/CT staging where unusually site for SCC such as liver was identified. CECT, contrast-enhanced computed tomography; CUP, carcinoma of unknown primary; FDG-PET/CT, fluorodeoxyglucose positron emission tomography/computed tomography; FNAC, fine needle aspiration cytology; MIP, maximum intensity projection; SCC, squamous cell carcinoma; USG, ultrasonography.

| Figure 3:A 33-year-old male, occasional smoker, presented with left-sided neck swelling. The biopsy from left level III lymph nodes turned out to be metastatic deposits of squamous carcinoma. He underwent 18F-FDG PET/CT to find primary and baseline staging. The FDG-PET/CT revealed metabolically active left-sided neck node (level V), multiple mediastinal nodes, right middle lobe paracardiac lesion, and abdominal nodes. Biopsy was attempted from chest lesion and mediastinal nodules, which revealed similar histology and other infective etiologies were ruled out. A diagnosis of metastatic SCC-CUP was made. The patient further received palliative chemotherapy as per protocol (paclitaxel and carboplatin). The response evaluation using FDG PET/CT showed response to cervical lesion but there was an increase in size and metabolism of other lesions, with multiple new lung nodules noted. The present therapy was stopped and molecular analysis was underway for the patient to decide further therapeutic options. (a) PET MIP images pre- and post-chemotherapy showing disease progression, (b) CT and PET/CT fused transverse section images pre- and post-chemotherapy at level V node showing complete resolution of the lesion. (c) CT and PET/CT fused coronal section images pre- and post-chemotherapy showing disease progression in abdominal and mediastinal nodes. (d) CT and PET/CT transverse section images pre- and post-chemotherapy showing progression of pleural-based nodule and newly seen tiny pulmonary nodules. The present illustration demonstrates the utility of FDG PET/CT in staging disease in CUP and response evaluation to systemic chemotherapy. CUP, carcinoma of unknown primary; 18F-FDG-PET/CT, fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography; MIP, maximum intensity projection; SCC, squamous cell carcinoma.

| Figure 4:A 62-year-old male presented with right hip and lower back pain for 3 months, MRI spine showed extensive marrow replacing lesion in the pelvic bones and vertebrae suggestive of metastatic deposits. FDG-PET/CT was considered to look for primary lesion and characterization of skeletal lesions, which showed FDG avid left lung mass which was suspected for primary lesion along with other tiny nodules. FDG avid metastatic appearing mediastinal, supraclavicular, and abdominal nodes were noted along with FDG avid lesions in the brain, liver, spleen, left kidney, right adrenal, and skeletal system (including multiple vertebrae, pelvis, sacrum, and bilateral femur). The USG-guided biopsy from left supra-clavicular node showed poorly differentiated non-small cell carcinoma. The disease was diagnosed as metastatic lung cancer with multiple soft tissue (including brain) and skeletal metastatic lesions. (a) FDG-PET/CT MIP images showing multiple regions of pathological skeletal and multiple other lesions scattered over the whole body. (b) CECT and PET/CT fused transverse section images, showing FDG concentrating left lung upper lobe mass and mediastinal nodes, enhancing lesion in right temporal lobe, and sagittal section image showing sclerotic lesions in multiple vertebrae and pelvic bones. The present illustration demonstrates the utility of FDG-PET/CT in guiding primary diagnosis and whole-body disease staging in evaluation of a patient presenting with metastatic skeletal disease. CECT, contrast-enhanced computed tomography; FDG-PET/CT, fluorodeoxyglucose positron emission tomography/computed tomography; MIP, maximum intensity projection; MRI, magnetic resonance imaging; USG, ultrasonography.

| Figure 5:A 60-year-old female presented with right upper abdomen discomfort and with USG suggestive of multiple mass lesions in the liver; patient got evaluated further with CECT scan and liver lesions were labelled not typical for HCC though they showed arterial enhancement with abdominal nodes. Her serum chromogranin A level was highly raised; thus, she was referred for 68Ga-DOTATATE PET/CT scan to ascertain the primary and also evaluate disease extent. 68Ga-DOTATATE PET/CT revealed SSTR-expressing mesenteric mass, abdominal nodes, liver lesions, and incidentally detected well-defined left lateral ventricular lesion in the brain, for which the patient was asymptomatic. A biopsy from the liver lesion revealed this to be grade I (Mib-1 index of 1%) neuroendocrine tumor. The disease was labelled as metastatic mesenteric NET with nodal and liver metastases and incidentally detected meningioma/? cerebral lesion. The patient was started on long-acting octreotide injections; she revealed stable and asymptomatic disease post 6 months. (a) 68Ga-DOTATATE PET/CT MIP image, showing SSTR-expressing mesenteric lesion, liver lesions (Krenning score 4), and left cerebral lesion. (b) CECT and PET/CT fused transaxial section images showing SSTR-expressing mesenteric lesion, liver lesions, and left cerebral (ventricular) lesion. The present illustration demonstrates the utility of SSTR-based PET/CT in guiding primary diagnosis, disease staging-grading, and guiding therapy in evaluation of NETs presenting with metastatic disease. CECT, contrast-enhanced computed tomography; HCC, hepatocellular carcinoma; NET, neuroendocrine tumor; PET/CT, positron emission tomography/computed tomography; SSTR, somatostatin receptor; USG, ultrasonography.

| Figure 6:A 34-year-old male presented with generalized weakness and abdominal discomfort, showed multiple liver lesions and splenomegaly on CECT. The liver biopsy revealed to be grade-II NET with Mib-1 index of 13%. He underwent baseline 68Ga-DOTATATE PET/CT scan to find primary and also disease extent. 68Ga-DOTATATE PET/CT revealed SSTR-expressing multiple bilobar liver lesions and sclerotic skeletal lesions; however, no obvious primary lesion could be found. The patient was diagnosed as grade II metastatic NET with unknown primary and being evaluated for Chemo-PRRT treatment protocol considering SSTR expressing (Krenning score 3) multiple liver and bone lesions. (a) 68Ga-DOTATATE PET/CT MIP image showing SSTR-expressing bilobar liver lesions (Kenning score 3) and multiple skeletal lesions. (b) CECT and PET/CT fused transverse section images showing SSTR-expressing liver lesions; sagittal section showing multiple sclerotic skeletal lesions. The present illustration demonstrates the utility of SSTR-based PET/CT in guiding diagnosis and disease staging in evaluation of CUP-NETs presenting with metastatic disease. CECT, contrast-enhanced computed tomography; MIP, maximum intensity projection; NET, neuroendocrine tumor; PET/CT, positron emission tomography/computed tomography; SSTR, somatostatin receptor;

| Flowchart 1:Screening, baseline diagnosis, and staging.

| Flowchart 2:Treatment response assessment in CUP with FDG-PET/CT.

| Flowchart 3:An algorithmic CUP-NET management strategy.

| Flowchart 4:Follow-up in CUP following completion of treatment.
References
- Varghese AM, Arora A, Capanu M. et al. Clinical and molecular characterization of patients with cancer of unknown primary in the modern era. Ann Oncol 2017; 28 (12) 3015-3021
- Rassy E, Nicolai P, Pavlidis N. Comprehensive management of HPV-related squamous cell carcinoma of the head and neck of unknown primary. Head Neck 2019; 41 (10) 3700-3711
- Hemminki K, Chen B, Kumar A. et al. Germline genetics of cancer of unknown primary (CUP) and its specific subtypes. Oncotarget 2016; 7 (16) 22140-22149
- Losa F, Iglesias L, Pané M. et al. 2018 consensus statement by the Spanish Society of Pathology and the Spanish Society of Medical Oncology on the diagnosis and treatment of cancer of unknown primary. Clin Transl Oncol 2018; 20 (11) 1361-1372
- Mathur P, Sathishkumar K, Chaturvedi M. et al; ICMR-NCDIR-NCRP Investigator Group. Cancer Statistics, 2020: Report From National Cancer Registry Programme, India. JCO Glob Oncol 2020; 6: 1063-1075
- Pavlidis N, Pentheroudakis G. Cancer of unknown primary site. Lancet 2012; 379 (9824): 1428-1435
- Rassy E, Pavlidis N. The currently declining incidence of cancer of unknown primary. Cancer Epidemiol 2019; 61: 139-141
- Qaseem A, Usman N, Jayaraj JS, Janapala RN, Kashif T. Cancer of unknown primary: a review on clinical guidelines in the development and targeted management of patients with the unknown primary site. Cureus 2019; 11 (09) e5552
- Maghami E, Ismaila N, Alvarez A. et al. Diagnosis and management of squamous cell carcinoma of unknown primary in the head and neck: ASCO guideline. J Clin Oncol 2020; 38 (22) 2570-2596
- Fizazi K, Greco FA, Pavlidis N, Daugaard G, Oien K, Pentheroudakis G. ESMO Guidelines Committee. Cancers of unknown primary site: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015; 26 (Suppl. 05) v133-v138
- Scarbrook A. Carcinoma of unknown origin. In: Nicolson T. ed. Recommendations of Cross Sectional Imaging in Cancer Management. London: Royal College of Radiologists; 2014
- Anbarasu A, Joshi V, Solanki R, Kumar J, Shah D. Head & Neck Imaging Sub Specialty Group Scanning Guidelines Ct Scan/Mr Imaging Version April; 2020. Accessed December 13, 2022 at: https://icri.iria.org.in/head-and-neck-imaging-guidelines-2020
- Boellaard R, Delgado-Bolton R, Oyen WJ. et al; European Association of Nuclear Medicine, (EANM). FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging 2015; 42 (02) 328-354
- Hope TA, Bergsland EK, Bozkurt MF. et al. Appropriate use criteria for somatostatin receptor PET imaging in neuroendocrine tumors. J Nucl Med 2018; 59 (01) 66-74
- Schöder H, Gönen M. Screening for cancer with PET and PET/CT: potential and limitations. J Nucl Med 2007; 48 (Suppl. 01) 4S-18S
- Kwee TC, Kwee RM. Combined FDG-PET/CT for the detection of unknown primary tumors: systematic review and meta-analysis. Eur Radiol 2009; 19 (03) 731-744
- Rusthoven KE, Koshy M, Paulino AC. The role of fluorodeoxyglucose positron emission tomography in cervical lymph node metastases from an unknown primary tumor. Cancer 2004; 101 (11) 2641-2649
- Lee JR, Kim JS, Roh JL. et al. Detection of occult primary tumors in patients with cervical metastases of unknown primary tumors: comparison of (18)F FDG PET/CT with contrast-enhanced CT or CT/MR imaging-prospective study. Radiology 2015; 274 (03) 764-771
- De Wever W, Meylaerts L, De Ceuninck L, Stroobants S, Verschakelen JA. Additional value of integrated PET-CT in the detection and characterization of lung metastases: correlation with CT alone and PET alone. Eur Radiol 2007; 17 (02) 467-473
- Park SB, Park JM, Moon SH. et al. Role of 18F-FDG PET/CT in patients without known primary malignancy with skeletal lesions suspicious for cancer metastasis. PLoS One 2018; 13 (05) e0196808
- Houshmand S, Salavati A, Segtnan EA, Grupe P, Høilund-Carlsen PF, Alavi A. Dual-time-point imaging and delayed-time-point fluorodeoxyglucose-PET/computed tomography imaging in various clinical settings. PET Clin 2016; 11 (01) 65-84
- Fei B, Schuster DM. PET molecular imaging-directed biopsy: a review. AJR Am J Roentgenol 2017; 209 (02) 255-269
- Sampathirao N, Basu S. MIB-1 index-stratified assessment of dual-tracer PET/CT with 68Ga-DOTATATE and 18F-FDG and multimodality anatomic imaging in metastatic neuroendocrine tumors of unknown primary in a PRRT workup setting. J Nucl Med Technol 2017; 45 (01) 34-41
- Fletcher JW, Djulbegovic B, Soares HP. et al. Recommendations on the use of 18F-FDG PET in oncology. J Nucl Med 2008; 49 (03) 480-508
- Barrio M, Czernin J, Fanti S. et al. The impact of somatostatin receptor-directed PET/CT on the management of patients with neuroendocrine tumor: a systematic review and meta-analysis. J Nucl Med 2017; 58 (05) 756-761
- Varadhachary, Gauri, James L.Abbruzzese. “Carcinoma of unknown primary.” Abeloff's Clinical oncology. Elsevier 2020; 1694-1702
- Gupta T, Master Z, Kannan S. et al. Diagnostic performance of post-treatment FDG PET or FDG PET/CT imaging in head and neck cancer: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging 2011; 38 (11) 2083-2095
- Slevin F, Subesinghe M, Ramasamy S, Sen M, Scarsbrook AF, Prestwich RJ. Assessment of outcomes with delayed (18)F-FDG PET-CT response assessment in head and neck squamous cell carcinoma. Br J Radiol 2015; 88 (1052): 20140592
- Marcus C, Ciarallo A, Tahari AK. et al. Head and neck PET/CT: therapy response interpretation criteria (Hopkins Criteria)-interreader reliability, accuracy, and survival outcomes. J Nucl Med 2014; 55 (09) 1411-1416
- Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med 2009; 50 (Suppl. 01) 122S-150S
- Cliffe H, Patel C, Prestwich R, Scarsbrook A. Radiotherapy response evaluation using FDG PET-CT-established and emerging applications. Br J Radiol 2017; 90 (1071): 20160764
- Martin RC, Fulham M, Shannon KF. et al. Accuracy of positron emission tomography in the evaluation of patients treated with chemoradiotherapy for mucosal head and neck cancer. Head Neck 2009; 31 (02) 244-250
- Evilevitch V, Weber WA, Tap WD. et al. Reduction of glucose metabolic activity is more accurate than change in size at predicting histopathologic response to neoadjuvant therapy in high-grade soft-tissue sarcomas. Clin Cancer Res 2008; 14 (03) 715-720
- Lopci E, Hicks RJ, Dimitrakopoulou-Strauss A. et al. Joint EANM/SNMMI/ANZSNM practice guidelines/procedure standards on recommended use of [18F]FDG PET/CT imaging during immunomodulatory treatments in patients with solid tumors version 1.0. Eur J Nucl Med Mol Imaging 2022; 49 (07) 2323-2341
- Ayati N, Sadeghi R, Kiamanesh Z, Lee ST, Zakavi SR, Scott AM. The value of 18F-FDG PET/CT for predicting or monitoring immunotherapy response in patients with metastatic melanoma: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging 2021; 48 (02) 428-448
- Wong ANM, McArthur GA, Hofman MS, Hicks RJ. The advantages and challenges of using FDG PET/CT for response assessment in melanoma in the era of targeted agents and immunotherapy. Eur J Nucl Med Mol Imaging 2017; 44 (Suppl. 01) 67-77
- Adnan A, Basu S. Discordance between histopathologic grading and dual-tracer PET/CT findings in metastatic NETs and outcome of 177Lu-DOTATATE PRRT: does in vivo molecular PET perform better from the viewpoint of prediction of tumor biology?. J Nucl Med Technol 2021; 50 (Suppl. 03) 248-255
- Ruhlmann V, Ruhlmann M, Bellendorf A. et al. Hybrid imaging for detection of carcinoma of unknown primary: a preliminary comparison trial of whole-body PET/MRI versus PET/CT. Eur J Radiol 2016; 85 (11) 1941-1947
- Kumar R, Singh SK, Mittal BR. et al. Safety and diagnostic yield of 68Ga prostate-specific membrane antigen PET/CT-guided robotic-assisted transgluteal prostatic biopsy. Radiology 2022; 303 (02) 392-398
- Kratochwil C, Flechsig P, Lindner T. et al. 68Ga-FAPI PET/CT: tracer uptake in 28 different kinds of cancer. J Nucl Med 2019; 60 (06) 801-805
- Gu B, Xu X, Zhang J. et al. The added value of 68Ga-FAPI PET/CT in patients with head and neck cancer of unknown primary with 18F-FDG-negative findings. J Nucl Med 2022; 63 (06) 875-881
- Niederkohr RD, Greenspan BS, Prior JO. et al. Reporting guidance for oncologic 18F-FDG PET/CT imaging. J Nucl Med 2013; 54 (05) 756-761


 PDF
PDF  Views
Views  Share
Share

