Imaging Recommendations for Diagnosis, Staging, and Management of Lung Cancer
CC BY 4.0 · Indian J Med Paediatr Oncol 2023; 44(02): 181-193
DOI: DOI: 10.1055/s-0042-1759572
Abstract
Globally and in India, lung cancer is one of the leading malignancies in terms of incidence and mortality. Smoking and environmental pollution are the common risk factors for developing lung cancer. Traditionally, lung cancer is divided into small cell and nonsmall cell types, with nonsmall cell carcinomas including squamous cell carcinoma, adenocarcinoma, and large cell carcinoma.
In this review article, we describe the imaging recommendations and findings in the diagnosis, staging, and management of lung cancer, including the imaging of treatment-related complications.
Publication History
Article published online:
24 January 2023
© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Globally and in India, lung cancer is one of the leading malignancies in terms of incidence and mortality. Smoking and environmental pollution are the common risk factors for developing lung cancer. Traditionally, lung cancer is divided into small cell and nonsmall cell types, with nonsmall cell carcinomas including squamous cell carcinoma, adenocarcinoma, and large cell carcinoma.
In this review article, we describe the imaging recommendations and findings in the diagnosis, staging, and management of lung cancer, including the imaging of treatment-related complications.
Keywords
cancer - imaging - lung - recommendationsIntroduction
Epidemiology
Globally, lung cancer is one of the leading malignancies in terms of incidence and mortality. It is the second most common malignancy in both the genders.[1] In India, lung cancer is one of the leading malignancies in men and fifth most common cancer in women. Its incidence varies between numerous regions of India. The projected incidence of lung cancer in men in India in 2020 was 9.9 per 100,000 people.
Risk Factors
Smoking (80–90%) and environmental pollution are the leading risk factors for lung cancer in India.[2] Exposure to carcinogens in genetically susceptible individuals may cause activation of oncogenes and/or inactivation of tumor suppressor genes, resulting in neoplastic proliferation of cells.
Etiopathogenesis
Traditionally, lung cancer is classified into small cell lung cancer (SCLC) and nonsmall cell lung cancer (NSCLC) types, with nonsmall cell carcinomas including squamous cell carcinoma, adenocarcinoma, and large cell carcinoma. There is an increasing incidence of NSCLC in women and nonsmokers. Following the worldwide trend, some studies in India are also reporting adenocarcinoma to be the most common pathological type in recent times, unlike squamous cell carcinoma that was more common previously.[2] However, other studies report no such change and still consider squamous cell carcinoma as the most common histopathological type.[3]
Clinical Presentation
Most patients with lung cancer in India are men (up to 82.9%); this disease is more commonly o b s e r v e d in the age range of 46 to 70 years, with a mean age of 58 years. The most commonly reported symptoms include cough, loss of weight and appetite, dyspnea, fatigue, chest pain, and hemoptysis. Patients may also present with paraneoplastic syndromes. Examination findings include digital clubbing, lymphadenopathy, superior vena cava obstruction, and neurological deficits in metastatic disease.[4] Although in countries which have adopted lung cancer screening could potentially be detecting lung cancers at an earlier stage, 44 to 47.6%-of patients with lung cancer in India present at an advanced stage with metastatic disease.[2]
Imaging plays an important role not only in the initial workup of patients with lung cancer but also in their regular follow-ups. It is also important to be aware of the changing profile of imaging appearances in patients with utilization of newer cancer treatments like immunotherapy.[5]
Imaging Referral Guidelines
General practitioners and other primary healthcare providers should refer a patient for a chest radiograph (CXR) when there are symptoms and signs suggestive of lung cancer like hemoptysis, loss of weight and appetite, persistent cough or dyspnea, chest pain, and abnormal chest signs. If the CXR is abnormal or if there is high index of clinical suspicion, even if CXR is normal, patient should be referred for a computed tomographic (CT) scan and to a specialist.[6]
On CXR, lung cancer may present as a nodule, irregular mass, nonresolving consolidation, lobar collapse, effusion, hilar or mediastinal enlargement. But, usually when these are observed on CXR, the disease stage is advanced. In early stages, CXR may be appear to be normal.
AJCC, NCCN Guidelines for Imaging in Lung Cancer[7] [8] [9]
The first imaging test to be performed in a suspected case of lung cancer is CXR. Contrast-enhanced CT (CECT) of chest and upper abdomen (including adrenal glands) is recommended for further evaluation. 18F-FDG PET-CT (positron emission tomography with 2-deoxy-2-[fluorine-18]fluoro- D- glucose integrating CT) is indicated in patients with no signs of metastatic spread on CT who are candidates for curative intent treatments.
Contrast-enhanced magnetic resonance imaging (MRI) of brain (or CECT of brain, if MRI not available) is recommended in patients with NSCLCs (stages Ib and higher) and small cell carcinomas (any stage) even if there are no relevant symptoms.
Contrast-enhanced MRI of thoracic inlet, spine, and brachial plexus is indicated to check for Pancoast tumor for the sake of local tumor extension assessment.
Indian Radiological and Imaging Association Guidelines for Imaging in Lung Cancer[10]
18F-FDG PET-CT scan is the most accurate investigation method for the staging of lung cancer. If a PET scan is not available, then a CECT scan of thorax, abdomen, pelvis, and whole-body bone scan needs to be done.
American College of Radiology Appropriateness Criteria for Imaging in Lung Cancer ([Table 1])[11]
|
CT thorax |
CECT is preferred. NCCT can be done if contrast cannot be administered for any reason |
|
18F-FDG PET-CT |
PET imaging from skull base to upper thigh is recommended for evaluation of extrathoracic disease |
|
CT abdomen and pelvis |
To look for extrathoracic disease if PET-CT is not available |
|
Bone scan |
To look for skeletal metastasis if PET-CT is not available |
|
MRI brain |
With gadolinium in NSCLC stage II, III, or IV even in the absence of neurological symptoms Any stage of NSCLC with neurological symptoms All small cell carcinoma (irrespective of stage) |
|
CT head |
With contrast, only if MRI brain cannot be performed |
|
MRI thorax |
For superior sulcus tumors or in equivocal findings on chest CT, to better evaluate for chest wall or mediastinal infiltration |
|
MRI abdomen |
Chemical shift imaging of adrenal gland lesions to see if they are adenomas or metastases (may not be necessary if PET-CT is performed) |
|
Organization |
Patient age/symptoms |
Smoking history (pack-years) |
Other factors |
|---|---|---|---|
|
ACCP, ASCO, ATS, ACS, and ALS |
55–74, asymptomatic |
≥ 30 |
Less than 15 years since smoking cessation |
|
NCCN |
55–74, asymptomatic or ≥ 50 asymptomatic |
≥ 30 ≥20 |
Less than 15 years since smoking cessation or one or more additional risk factors like pulmonary disease, family history of lung cancer, personal cancer history, radon exposure |
|
ESR |
55–80 years |
At least 30 pack-years |
Current smoker or exsmoker who has quit smoking within the last 15 years |
|
USPSFT |
55–80 asymptomatic |
≥ 30 |
Less than 15 years since smoking cessation |
|
kV |
120 |
|
mAs |
50 |
|
Pitch |
1.2 |
|
Slice thickness |
1 mm |
|
Slice interval |
0.5 mm |
|
Scan region |
Thoracic inlet to costophrenic angles |
|
Respiration |
Suspended end-inspiration |
|
Reconstructions |
Lung (e.g., B70) and soft tissue (e.g., B35) reconstructions and window setting |
|
Effective radiation dose |
< 1> |
|
Imaging features |
Benign |
Malignant |
Comments |
|---|---|---|---|
|
Less than 1 cm |
More than 3 cm |
Average of two orthogonal measurements is taken Smaller lesions can also be malignant |
|
|
Sharp, smooth, or well-defined |
Spiculated, irregular, ill-defined, “pleural tail” |
20%-of malignant nodules may show smooth and well-defined margins |
|
|
Internal morphology |
|||
|
Pure ground glass density nodules <5> |
- Pure ground glass density nodule 6–30 mm could represent adenocarcinoma in situ - Part-solid nodule with solid component < 5 mm could represent minimally invasive adenocarcinoma - Part-solid nodule with solid component > 5 mm could represent invasive adenocarcinoma lepidic predominant adenocarcinoma - Solid nodules or consolidation could represent mucinous adenocarcinoma in situ, invasive mucinous adenocarcinoma |
Based on density nodules can be solid or subsolid. Subsolid nodules can be pure ground glass density or part-solid |
|
|
Calcifications |
Diffuse, central, popcorn, laminated |
Amorphous, punctate |
|
|
Fat[28] |
Presence of fat (−40 to −120 HU) favors benign lesion like hamartoma |
Fat is usually absent in malignancy |
|
|
Cavitation |
Smooth, thin walls. Usually less than 4 mm wall thickness |
Thick, irregular walls. Usually more than 16 mm wall thickness |
|
|
Growth rate[29] |
More than 400 days or very short in infections |
Doubling time 20 to 400 days (average 240) |
The serial volumes instead of diameters and computer-aided doubling of volume of small nodules have been proposed to be an accurate way to evaluate growth Small and large cell cancers may show a faster growth |
|
Contrast enhancement[30] |
Less than 15 HU |
More than 20 HU |
Larger malignant lesions tend to show more heterogeneous contrast enhancement due to presence of necrosis within the lesion |
|
Metabolism[31] |
Less than 2.5 SUV |
More than 2.5 SUV |
Note that infective and inflammatory conditions also can show high SUV. PET can help in guiding biopsy to the areas with increased FDG avidity |
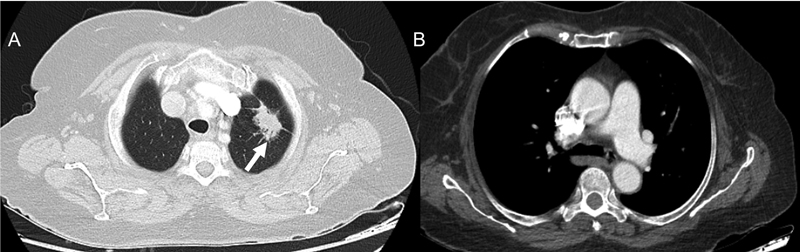
| Figure 1:(A) Axial computed tomographic (CT) thorax section in lung window shows a spiculated mass (white arrow) of longest dimension 2.8 cm in the apicoposterior segment of the left upper lobe. (B) Axial CT thorax section in mediastinal window reveals that there are no enlarged mediastinal or hilar nodes. TNM (tumor , nodes, and metastases) stage: T1cN0M0.
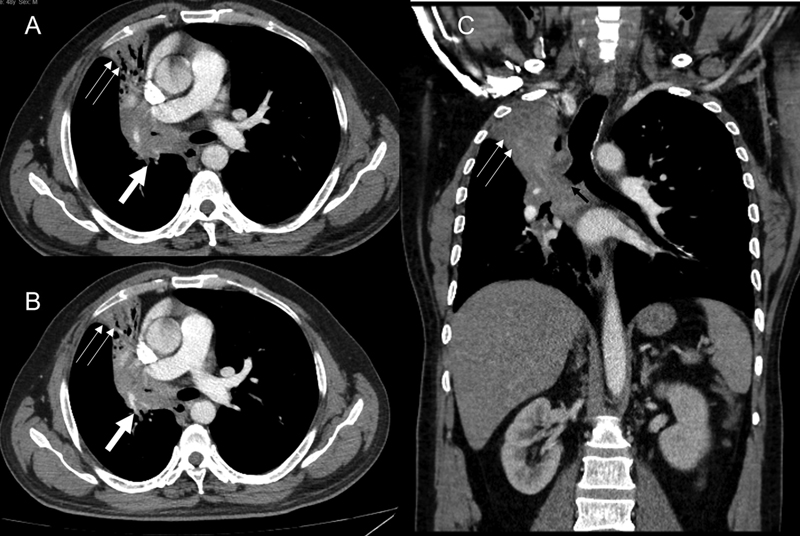
| Figure 2:(A and B) Axial computed tomographic (CT) thorax sections demonstrate central hilar mass having convex margin (white arrow) causing occlusion of right upper lobe bronchus, bronchus intermedius, right middle lobe bronchus with the resultant collapse, with mild peripheral bronchiectasis of right upper and right middle lobe having retained secretions in the peripheral bronchi (double thin white arrow). The lesion infiltrates and merges with the ipsilateral mediastinal, hilar, and subcarinal nodes. (C) The coronal CT thorax section also reveals the medial extension into the right main bronchus (black arrow). No distant metastases. TNM stage: T4N2.
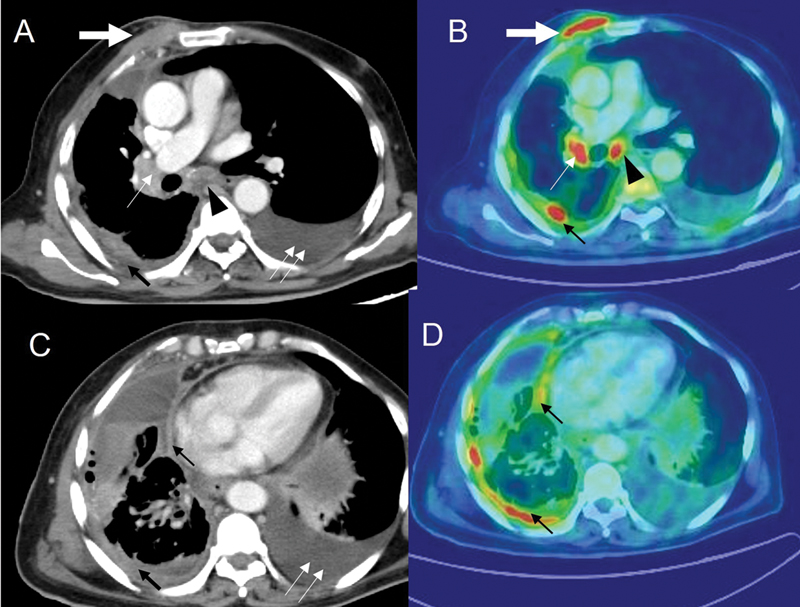
| Figure 3:Patient with adenocarcinoma of the lung with positron emission tomography-computed tomography (PET-CT) imaging. CT thorax (A and C) and corresponding flourine-18 fluorodeoxyglucose positron emission tomography (18F-FDG PET) images (B and D) demonstrate chest wall extension (thick white arrow), having standardized uptake value (SUV) of 9.4, enlarged right hilar (thin white arrow), and subcarinal node (black arrowhead) with SUV of 8.4 and 7.6, respectively. Pleural effusion with nodular pleural thickening (thin black arrow) with SUV of 6.6. Moderate amount of pleural effusion (double thin white arrows) on the left side.
|
T staging |
N staging |
M staging |
|---|---|---|
|
T0—No primary tumor Tis—Carcinoma in situ T1—Tumor < 3 cm (T1mi—minimally invasive adenocarcinoma; T1a—Superficial spreading tumor in central airways) T1a—Tumor < 1 cm T1b—Tumor > 1 cm but < 2 cm T1c—Tumor > 2 cm but < 3 cm T2—Tumor >3 cm but < 5 cm or tumor involving visceral pleura/main bronchus (not carina)/atelectasis up to hilum T2a—Tumor > 3 cm but < 4 cm T2b—Tumor > 4 cm but < 5 cm T3—Tumor > 5 cm but < 7 cm or invading chest wall, pericardium, phrenic nerve; or separate tumor nodule(s) in the same lobe T4—Tumor > 7 cm or tumor invading mediastinum/diaphragm/hear/great vessels/recurrent laryngeal nerve/carina/trachea/esophagus/spine or tumor nodule(s) in a different lobe in same lung |
N0—No regional node metastasis N1—Metastasis in ipsilateral pulmonary or hilar nodes N2—Metastasis in ipsilateral mediastinal or subcarinal nodes N3—Metastasis in contralateral mediastinal, hilar, or supraclavicular nodes |
M0—No distant metastasis M1a—Malignant pleural or pericardial effusion or pleural or pericardial nodules or separate tumor nodule(s) in the contralateral lung M1b—Single extrathoracic metastasis M1c—Multiple extrathoracic metastases (in 1 or >1 organ) |
|
T/M |
Subcategory |
N0 |
N1 |
N2 |
N3 |
|---|---|---|---|---|---|
|
T1 |
T1a |
IA1 |
IIB |
IIIA |
IIIB |
|
T1b |
IA2 |
IIB |
IIIA |
IIIB |
|
|
T1c |
IA3 |
IIB |
IIIA |
IIIB |
|
|
T2 |
T2a |
IB |
IIB |
IIIA |
IIIB |
|
T2b |
IIA |
IIB |
IIIA |
IIIB |
|
|
T3 |
T3 |
IIB |
IIIA |
IIIB |
IIIC |
|
T4 |
T4 |
IIIA |
IIIA |
IIIB |
IIIC |
|
M1 |
M1a |
IVA |
IVA |
IVA |
IVA |
|
M1b |
IVA |
IVA |
IVA |
IVA |
|
|
M1c |
IVB |
IVB |
IVB |
IVB |
|
Measurable and target lesions |
A maximum of 5 total target lesions (a maximum of 2 per organ) are quantitatively assessed The lesion must be at least 10 mm in size The sum of the longest diameters is recorded as the sum of measurable lesions (SOM) Pathological lymph nodes with a short axis diameter of 15 mm or more can be considered as target lesions. For lymph nodes, a short axis dimension is taken for SOM |
|
Nontarget and nonmeasurable lesions |
May be recorded qualitatively Lesions less than 10 mm; nodes between 10 and 15 mm Nonmeasurable lesions like pleural or pericardial effusion or lymphangitis |
|
Progressive disease (PD) |
At least 20%-increase in size over the nadir (smallest measured tumor burden during the course of the disease). The minimum increase should be at least 5 mm “Unequivocal” progression in nonmeasurable or other non-target lesions (effusions, bone lesions) is recorded as PD Any new lesions are considered as PD |
|
Partial response (PR) |
When there is more than 30%-decrease in size from baseline |
|
Complete response (CR) |
When there is complete disappearance of the lesion and pathological lymph nodes are reduced to less than 10 mm |
|
Stable disease (SD) |
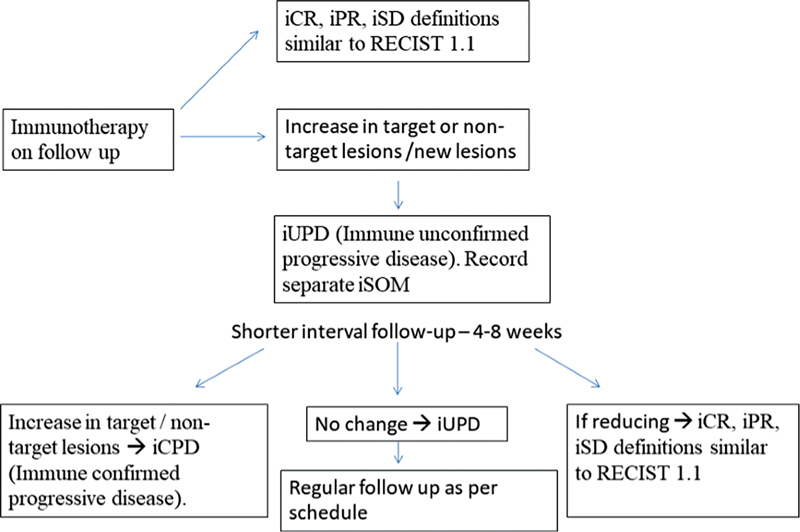
| Flowchart 1:iRECIST criteria for follow-up of patients who are on immunotherapy. iCR, immune complete response; iPR, immune partial response; iSD, immune stable disease.0.
Complications of Treatment
Complications of Radiotherapy
Conformal RT is typically used for patients with advanced stage lung cancer and the treatment course is over 6 to 7 weeks. In contrast, stereotactic body radiotherapy (SBRT) is used for early-stage lung cancer; precise high dose fractions are given over a period of 2 weeks.
Intensity-modulated RT (IMRT) is a technique in which radiation is delivered using multiple RT fields of varying influence; both conventional RT and SBRT can be delivered using this technique. Use of IMRT reduces the radiation delivered to the normal lung tissue around the tumor. Thus, treatment with IMRT can result in unusual patterns of pneumonitis in the patient, since radiological pneumonitis changes correlates with the shape of the RT field/plan.[37]
Acute radiation pneumonitis ([Fig. 4]) can manifest as consolidation with air-bronchogram and straight borders and occurs 1 to 6 months after RT. Radiation fibrosis sets in at 6 to 12 months and stabilizes at 1 year period. Radiation fibrosis manifests as traction bronchiectasis, volume loss, kinking of airways, and pleural thickening/effusion.
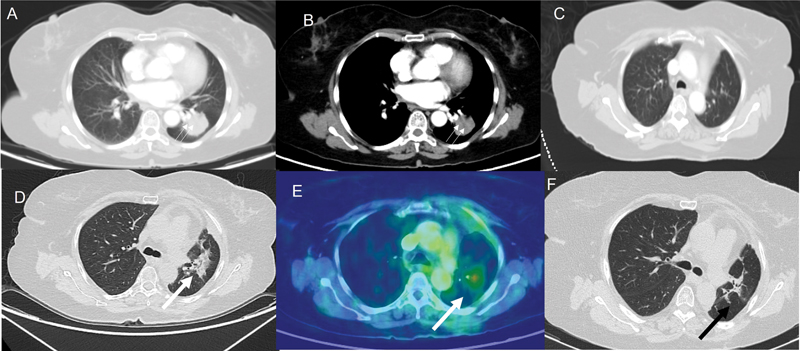
| Figure 4:(A and B) Computed tomographic (CT) thorax lung and mediastinal sections, respectively, demonstrate a lobulated mass (double thin white arrow) of 3.3 cm in the left lower lobe. No enlarged nodes. The patient underwent a left lower lobectomy. Histopathological examination revealed pleomorphic carcinoma with adenocarcinomatous component. Final stage was pT2aN2Mx. The patient had four cycles of adjuvant cisplatin and pemetrexed. (C) Post-chemotherapy and prior to radiotherapy, the CT thorax performed demonstrates no lesion in the left upper lobe. (D and E) CT thorax and corresponding flourine-18 fluorodeoxyglucose positron emission tomography images demonstrate an irregular lesion (thick white arrow) in the left upper lobe having an standardized uptake value of 4.6. As the lesion appeared after starting radiotherapy and as there was no evidence of infection clinically, radiation pneumonitis was considered the most likely diagnosis. The patient was treated with steroids and follow-up CT thorax (F) demonstrates the replacement of the irregular parenchymal lesion with small residual fibrotic changes (black arrow).
Post RT changes in the lung can be FDG-avid for up to 2 years. In previously irradiated patients, treatment with immune checkpoint inhibitors (ICI) can cause RT recall pneumonitis (pneumonitis in the irradiated regions of the lung), years after completion of RT. Besides pneumonitis, pulmonary artery thrombosis, esophagitis, fistulas, cardiac disease have been reported as complications of RT. Unlike radiation fibrosis, if the lesion is progressively increasing in size in real time or after 1 year of treatment, when the margins are convex and bulging, there is opacification of previously seen bronchograms, or development of new adenopathy of pleural effusion, then recurrence or disease progression needs to be suspected.[38]
Immune-Related Adverse Events[5] [39] [40]
Because of their unique mechanism of action, ICI can cause toxicities in several organs in the body, which can occur earlier or later during therapy. RT recall pneumonitis, described in post RT patients on ICI, occurs in previously irradiated tissue after weeks, months, or even years of radiation therapy.
Hypophysitis, sarcoid-like granulomatosis, lymphadenopathy, pneumonitis, hepatitis, colitis, adrenalitis, and inflammation in other parts of the body have been described in association with ICI toxicity. They must be differentiated from metastasis, infection, or progressive disease.
ICI pneumonitis is relatively rare but clinically serious with different patterns described. ([Table 8]).[41] [42] Treatment generally consists of corticosteroid therapy and delaying/stopping immunotherapy. After an initial response to corticosteroids, “Pneumonitis flare” can occur even without restarting immunotherapy.[43]
|
Cryptogenic organizing pneumonia (COP) pattern |
Peripherally distributed multifocal bilateral parenchyma consolidation and ground glass opacity (GGO) |
This is the most common pattern |
|
Diffuse alveolar damage pattern (DAD)(AIP/ARDS) (diffuse alveolar damage/acute interstitial pneumonia/acute respiratory distress syndrome) |
Diffuse/multifocal; GGO/consolidation with lung volume loss and traction bronchiectasis |
This is clinically the most serious type |
|
NSIP (nonspecific interstitial pneumonitis) pattern |
Peripherally distributed reticular opacities and GGO ± volume loss/traction bronchiectasis |
These patients have relatively mild clinical manifestations |
|
HP (hypersensitivity pneumonitis) pattern |
Diffuse centrilobular nodules and GGO ± air trapping |
|
ASCO 2019, lung cancer surveillance guidelines after definitive curative-intent therapy (stage I–III) |
|||
|---|---|---|---|
|
NSCLC |
SCLC |
Imaging modality |
|
|
1. Frequency of imaging |
Every 6 months for 2 years |
Every 6 months for 2 years |
Chest CT including adrenals ± contrast (contrast preferred) |
|
Annually after 2 years |
Annually after 2 years |
Low-dose screening chest CT |
|
|
2. Role of brain MRI |
No |
First 2 years—every 3 months during first year and every 6 months during second year in patients with or without PCI |
|
|
3. Role of circulating biomarkers or FDG-PET-CT |
No |
No |
|
|
4. Any patient factors precluding surveillance? |
May be omitted in clinically unsuitable patients or those unwilling to undergo further treatment. Age should not preclude surveillance imaging. Overall performance status, medical history, and patient preferences are to be considered. |
||
|
Stage |
Primary treatment |
Imaging modality |
Frequency of imaging |
|---|---|---|---|
|
I–II |
Surgery ± chemotherapy |
H&P and chest CT ± contrast |
Every 6 months for 2–3 years |
|
Low dose noncontrast chest CT |
Annually after 2–3 years |
||
|
I–II or III or IV (oligometastatic with all sites treated with definitive intent) |
Primary treatment included RT |
H&P and chest CT1 ± contrast |
Every 3–6 months for 3 years, then |
|
H&P and chest CT1 ± contrast |
Every 6 months for 2 years, then |
||
|
H&P and a low dose noncontrast CT chest |
annually |
||
|
Any residual or new radiographic abnormalities |
More frequent imaging may be required |
||
|
Any stage |
Chemotherapy ± RT—any residual or new radiographic abnormalities—more frequent imaging may be required. |
||
|
PET/CT or brain MRI |
Not routinely indicated. PET-CT may be useful where CT scan shows a mass to differentiate between malignancy versus radiation fibrosis, atelectasis, or other benign conditions2 |
||
|
Smoking cessation |
Smoking cessation advice, counselling, and pharmacotherapy |
||
|
Long term follow-up—cancer survivorship care |
Cancer surveillance as above, immunizations, health monitoring, counselling for health promotion, and wellness |
||
|
Frequency and modality of imaging |
H&P, blood work as clinically indicated. Chest CT ± abdomen/pelvis - Every 2–6 months (more frequently) for 2 years and less frequently thereafter |
|
Brain MRI (preferred) or brain CT with contrast |
Every 3–4 months during 1st year for all patients and then every 6 months during 2nd year, regardless of the PCI status |
|
New pulmonary nodule |
Workup for a potential new primary lesion should be initiated |
|
FDG-PET-CT |
Not recommended for routine follow-up |
|
Smoking cessation intervention |
For all patients with SCLC to decrease the occurrence of second primary tumors |
|
Cancer survivorship care |
Cancer surveillance as above, immunizations, health monitoring, counseling for health promotion and wellness |
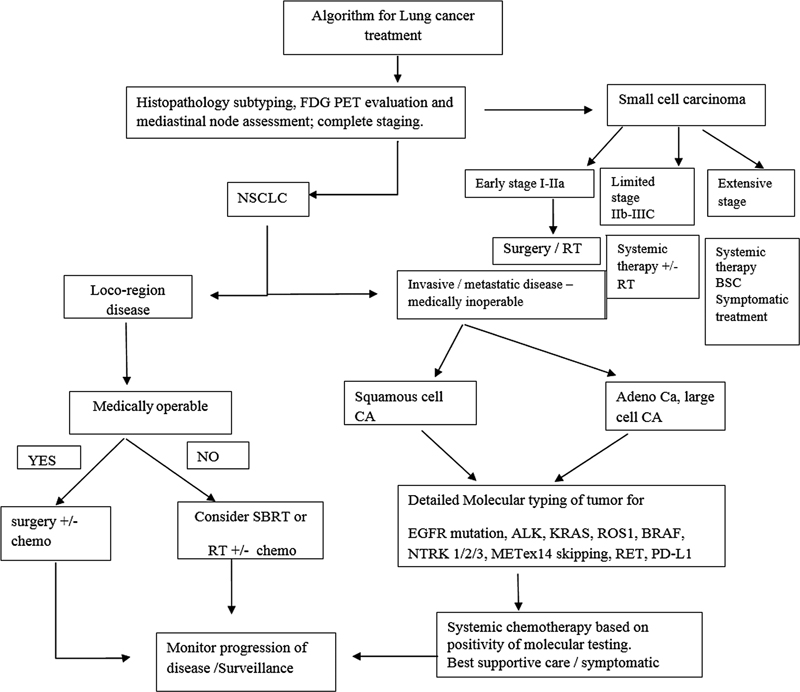
| Flowchart 2:Highlights of principles of management of lung cancer. FDG PET, flourine-18 fluorodeoxyglucose positron emission tomography; NSCLC, nonsmall cell lung cancer; SBRT, stereotactic body radiotherapy; EGFR, epidermal growth factor receptor.
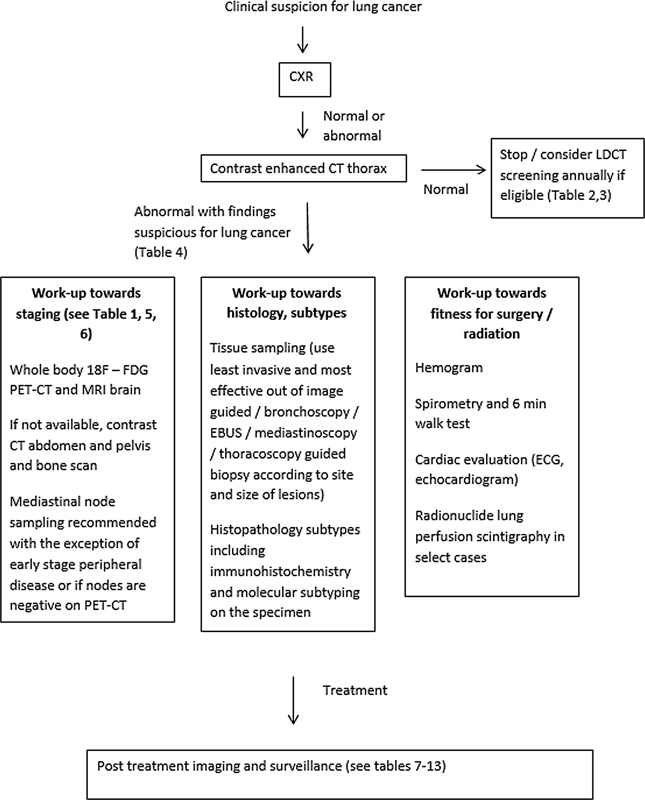
| Flowchart 3:Summary of recommendations in workup and follow-up of patients with lung cancer. CXR, chest X-ray; LDCT, low-dose computed tomography; EBUS, endobronchial ultrasound; ECG, electrocardiogram; 18F-FDG PET-CT, flourine-18 fluorodeoxyglucose positron emission tomography computed tomography.
Image-guided thermal ablation treatment (IGTA) is a local ablative therapy that can be undertaken by interventional radiologist after an multidisciplinary team meeting decision is made to treat the patient with IGTA. Most often this treatment is reserved for localized disease with tumor size less than 3 cm in size and potentially operable, but other comorbidities preclude surgery. Various electromagnetic energies like radio frequency, microwave, or cryoablation are used to thermally ablate the lesion without affecting the adjacent normal lung tissue. One of the common complications of IGTA is pneumothorax.
Follow-Up Imaging and Management of Recurrent Disease Including Specific Interventional and Palliative Measures
[Tables 12] and [13] highlight the salient points in follow-up and management of recurrent disease.[9]
|
Symptoms/site of recurrence |
Treatment options |
Follow-up imaging |
|---|---|---|
|
Localized symptoms |
Palliative RT |
No specific imaging recommended |
|
Diffuse brain metastases |
Palliative RT |
|
|
Skeletal metastases |
Surgical stabilization/palliative RT/bisphosphonates |
|
|
Limited metastases |
Resection |
|
|
Disseminated metastases |
RT/ systemic therapy |
References
- Thandra KC, Barsouk A, Saginala K, Aluru JS, Barsouk A. Epidemiology of lung cancer. Contemp Oncol (Pozn) 2021; 25 (01) 45-52
- Mohan A, Garg A, Gupta A. et al. Clinical profile of lung cancer in North India: a 10-year analysis of 1862 patients from a tertiary care center. Lung India 2020; 37 (03) 190-197
- Noronha V, Pinninti R, Patil VM, Joshi A, Prabhash K. Lung cancer in the Indian subcontinent. South Asian J Cancer 2016; 5 (03) 95-103
- Mathur P, Sathishkumar K, Chaturvedi M. et al; ICMR-NCDIR-NCRP Investigator Group. Cancer statistics, 2020: report from National Cancer Registry Programme, India. JCO Glob Oncol 2020; 6: 1063-1075
- Nishino M, Hatabu H, Hodi FS. Imaging of cancer immunotherapy: current approaches and future directions. Radiology 2019; 290 (01) 9-22
- Del Giudice ME, Young SM, Vella ET. et al. Guideline for referral of patients with suspected lung cancer by family physicians and other primary care providers. Can Fam Physician 2014; 60 (08) 711-716 , e376–e382
- Ganti AKP, Loo BWJ, Bassetti M. et al. Small cell lung cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2021; 19 (12) 1441-1464
- Ettinger DS, Wood DE, Aisner DL. et al. Non-small cell lung cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2022; 20 (05) 497-530
- Ettinger DS, Wood DE, Aisner DL. et al. NCCN guidelines insights: non-small cell lung cancer, Version 2.2021. J Natl Compr Canc Netw 2021; 19 (03) 254-266
- Accessed November 15, 2022, at: https://iria.org.in/radiology-imaging-guidelines
- de Groot PM, Chung JH, Ackman JB. et al; Expert Panel on Thoracic Imaging. ACR Appropriateness Criteria® noninvasive clinical staging of primary lung cancer. J Am Coll Radiol 2019; 16 (5S): S184-S195
- Melosky B, Blais N, Cheema P. et al. Standardizing biomarker testing for Canadian patients with advanced lung cancer. Curr Oncol 2018; 25 (01) 73-82
- Schmidt-Hansen M, Baldwin DR, Hasler E, Zamora J, Abraira V, Roqué I Figuls M. PET-CT for assessing mediastinal lymph node involvement in patients with suspected resectable non-small cell lung cancer. Cochrane Database Syst Rev 2014; (11) CD009519 DOI: 10.1002/14651858.CD009519.pub2.
- Brunelli A, Kim AW, Berger KI, Addrizzo-Harris DJ. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143 (5, Suppl): e166S-e190S
- American Cancer Society. Cancer facts & figures 2020. Accessed November 15, 2022, at: https://www.cancer.org/research/cancer-factsstatistics/all-cancer-facts-figures/cancer-facts-figures-2020.html
- Aberle DR, Adams AM, Berg CD. et al; National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365 (05) 395-409
- Henschke CI, McCauley DI, Yankelevitz DF. et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet 1999; 354 (9173): 99-105
- De Koning H, Van Der Aalst C, Ten Haaf K. et al. Effects of volume CT lung cancer screening: mortality results of the NELSON randomized-controlled population-based trial. Paper presented at: 2018 World Conference on Lung Cancer. Abstract PL02.05; 2018
- Kim H, Kim HY, Goo JM, Kim Y. Lung cancer CT screening and lung-RADS in a tuberculosis-endemic country: The Korean Lung Cancer Screening Project (K-LUCAS. Radiology 2020; 296 (01) 181-188
- Yang P. MS16.04 National Lung Screening Program in Taiwan. IASLC 19th World Conf . Lung Cancer 2018; 13 (10, Supplement): S274-S275
- Parang S, Bhavin J. LDCT screening in smokers in India-a pilot, proof-of-concept study. Indian J Radiol Imaging 2021; 31 (02) 318-322
- McKee BJ, Regis SM, McKee AB, Flacke S, Wald C. Performance of ACR lung-RADS in a clinical CT lung screening program. J Am Coll Radiol 2016; 13 (2, Suppl): R25-R29
- Wiener RS, Gould MK, Arenberg DA. et al; ATS/ACCP Committee on Low-Dose CT Lung Cancer Screening in Clinical Practice. An official American Thoracic Society/American College of Chest Physicians policy statement: implementation of low-dose computed tomography lung cancer screening programs in clinical practice. Am J Respir Crit Care Med 2015; 192 (07) 881-891
- Wood DE, Kazerooni EA, Baum SL. et al. Lung Cancer Screening, Version 3.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2018; 16 (04) 412-441
- Kauczor HU, Baird AM, Blum TG. et al; European Society of Radiology (ESR) and the European Respiratory Society (ERS). ESR/ERS statement paper on lung cancer screening. Eur Radiol 2020; 30 (06) 3277-3294
- Nitz JA, Erkmen CP. New 2021 USPSTF lung cancer screening criteria-an opportunity to mitigate racial disparity. JAMA Oncol 2022; 8 (03) 383-384
- Kishi K, Homma S, Kurosaki A. et al. Small lung tumors with the size of 1cm or less in diameter: clinical, radiological, and histopathological characteristics. Lung Cancer 2004; 44 (01) 43-51
- Bueno J, Landeras L, Chung JH. Updated Fleischner Society Guidelines for Managing Incidental Pulmonary Nodules: common questions and challenging scenarios. Radiographics 2018; 38 (05) 1337-1350
- Lacasse Y, Wong E, Guyatt GH, Cook DJ. Transthoracic needle aspiration biopsy for the diagnosis of localised pulmonary lesions: a meta-analysis. Thorax 1999; 54 (10) 884-893
- Silvestri GA, Gonzalez AV, Jantz MA. et al. Methods for staging non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143 (5, Suppl): e211S-e250S
- Birim O, Kappetein AP, Stijnen T, Bogers AJ. Meta-analysis of positron emission tomographic and computed tomographic imaging in detecting mediastinal lymph node metastases in nonsmall cell lung cancer. Ann Thorac Surg 2005; 79 (01) 375-382
- Amin MB. Ed. AJCC Cancer Staging Manual 8th ed. Cham, Switzerland: Springer; 2017: 431-455
- Kalemkerian GP, Gadgeel SM. Modern staging of small cell lung cancer. J Natl Compr Canc Netw 2013; 11 (01) 99-104
- Eisenhauer EA, Therasse P, Bogaerts J. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45 (02) 228-247
- Schwartz LH, Litière S, de Vries E. et al. RECIST 1.1-update and clarification: from the RECIST committee. Eur J Cancer 2016; 62: 132-137
- Seymour L, Bogaerts J, Perrone A. et al; RECIST working group. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 2017; 18 (03) e143-e152
- Strange CD, Shroff GS, Truong MT, Nguyen Q-N, Vlahos I, Erasmus JJ. Imaging of the post-radiation chest in lung cancer. Clin Radiol 2022; 77 (01) 19-30
- Huang K, Senthi S, Palma DA. et al. High-risk CT features for detection of local recurrence after stereotactic ablative radiotherapy for lung cancer. Radiother Oncol 2013; 109 (01) 51-57
- Berghmans T, Dingemans AM, Hendriks LEL, Cadranel J. Immunotherapy for nonsmall cell lung cancer: a new therapeutic algorithm. Eur Respir J 2020; 55 (02) 1901907 DOI: 10.1183/13993003.01907-2019.
- Nishino M, Tirumani SH, Ramaiya NH, Hodi FS. Cancer immunotherapy and immune-related response assessment: the role of radiologists in the new arena of cancer treatment. Eur J Radiol 2015; 84 (07) 1259-1268
- Nishino M, Sholl LM, Hodi FS, Hatabu H, Ramaiya NH. Anti-PD-1-related pneumonitis during cancer immunotherapy. N Engl J Med 2015; 373 (03) 288-290
- Nishino M. Imaging of oncologic treatment-related pneumonitis: a focused review on emerging issues of immune checkpoint inhibitor pneumonitis, from the AJR special series on inflammation. AJR Am J Roentgenol 2022; 218 (01) 19-27
- Nishino M, Ramaiya NH, Awad MM. et al. PD-1 inhibitor-related pneumonitis in advanced cancer patients: radiographic patterns and clinical course. Clin Cancer Res 2016; 22 (24) 6051-6060
- Johkoh T, Lee KS, Nishino M. et al. Chest CT diagnosis and clinical management of drug-related pneumonitis in patients receiving molecular targeting agents and immune checkpoint inhibitors: a position paper from the Fleischner Society. Radiology 2021; 298 (03) 550-566
- Schneider BJ, Ismaila N, Aerts J. et al. Lung cancer surveillance after definitive curative-intent therapy: ASCO guideline. J Clin Oncol 2020; 38 (07) 753-766
- Maconachie R, Mercer T, Navani N, McVeigh G. Guideline Committee. Lung cancer: diagnosis and management: summary of updated NICE guidance. BMJ 2019; 364: l1049 DOI: 10.1136/bmj.l1049.
- Choi JI. Medically inoperable stage I non-small cell lung cancer: best practices and long-term outcomes. Transl Lung Cancer Res 2019; 8 (01) 32-47
Address for correspondence
Publication History
Article published online:
24 January 2023
© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Figure 1:(A) Axial computed tomographic (CT) thorax section in lung window shows a spiculated mass (white arrow) of longest dimension 2.8 cm in the apicoposterior segment of the left upper lobe. (B) Axial CT thorax section in mediastinal window reveals that there are no enlarged mediastinal or hilar nodes. TNM (tumor , nodes, and metastases) stage: T1cN0M0.

| Figure 2:(A and B) Axial computed tomographic (CT) thorax sections demonstrate central hilar mass having convex margin (white arrow) causing occlusion of right upper lobe bronchus, bronchus intermedius, right middle lobe bronchus with the resultant collapse, with mild peripheral bronchiectasis of right upper and right middle lobe having retained secretions in the peripheral bronchi (double thin white arrow). The lesion infiltrates and merges with the ipsilateral mediastinal, hilar, and subcarinal nodes. (C) The coronal CT thorax section also reveals the medial extension into the right main bronchus (black arrow). No distant metastases. TNM stage: T4N2.

| Figure 3:Patient with adenocarcinoma of the lung with positron emission tomography-computed tomography (PET-CT) imaging. CT thorax (A and C) and corresponding flourine-18 fluorodeoxyglucose positron emission tomography (18F-FDG PET) images (B and D) demonstrate chest wall extension (thick white arrow), having standardized uptake value (SUV) of 9.4, enlarged right hilar (thin white arrow), and subcarinal node (black arrowhead) with SUV of 8.4 and 7.6, respectively. Pleural effusion with nodular pleural thickening (thin black arrow) with SUV of 6.6. Moderate amount of pleural effusion (double thin white arrows) on the left side.

| Flowchart 1:iRECIST criteria for follow-up of patients who are on immunotherapy. iCR, immune complete response; iPR, immune partial response; iSD, immune stable disease.0.

| Figure 4:(A and B) Computed tomographic (CT) thorax lung and mediastinal sections, respectively, demonstrate a lobulated mass (double thin white arrow) of 3.3 cm in the left lower lobe. No enlarged nodes. The patient underwent a left lower lobectomy. Histopathological examination revealed pleomorphic carcinoma with adenocarcinomatous component. Final stage was pT2aN2Mx. The patient had four cycles of adjuvant cisplatin and pemetrexed. (C) Post-chemotherapy and prior to radiotherapy, the CT thorax performed demonstrates no lesion in the left upper lobe. (D and E) CT thorax and corresponding flourine-18 fluorodeoxyglucose positron emission tomography images demonstrate an irregular lesion (thick white arrow) in the left upper lobe having an standardized uptake value of 4.6. As the lesion appeared after starting radiotherapy and as there was no evidence of infection clinically, radiation pneumonitis was considered the most likely diagnosis. The patient was treated with steroids and follow-up CT thorax (F) demonstrates the replacement of the irregular parenchymal lesion with small residual fibrotic changes (black arrow).

| Flowchart 2:Highlights of principles of management of lung cancer. FDG PET, flourine-18 fluorodeoxyglucose positron emission tomography; NSCLC, nonsmall cell lung cancer; SBRT, stereotactic body radiotherapy; EGFR, epidermal growth factor receptor.

| Flowchart 3:Summary of recommendations in workup and follow-up of patients with lung cancer. CXR, chest X-ray; LDCT, low-dose computed tomography; EBUS, endobronchial ultrasound; ECG, electrocardiogram; 18F-FDG PET-CT, flourine-18 fluorodeoxyglucose positron emission tomography computed tomography.
References
- Thandra KC, Barsouk A, Saginala K, Aluru JS, Barsouk A. Epidemiology of lung cancer. Contemp Oncol (Pozn) 2021; 25 (01) 45-52
- Mohan A, Garg A, Gupta A. et al. Clinical profile of lung cancer in North India: a 10-year analysis of 1862 patients from a tertiary care center. Lung India 2020; 37 (03) 190-197
- Noronha V, Pinninti R, Patil VM, Joshi A, Prabhash K. Lung cancer in the Indian subcontinent. South Asian J Cancer 2016; 5 (03) 95-103
- Mathur P, Sathishkumar K, Chaturvedi M. et al; ICMR-NCDIR-NCRP Investigator Group. Cancer statistics, 2020: report from National Cancer Registry Programme, India. JCO Glob Oncol 2020; 6: 1063-1075
- Nishino M, Hatabu H, Hodi FS. Imaging of cancer immunotherapy: current approaches and future directions. Radiology 2019; 290 (01) 9-22
- Del Giudice ME, Young SM, Vella ET. et al. Guideline for referral of patients with suspected lung cancer by family physicians and other primary care providers. Can Fam Physician 2014; 60 (08) 711-716 , e376–e382
- Ganti AKP, Loo BWJ, Bassetti M. et al. Small cell lung cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2021; 19 (12) 1441-1464
- Ettinger DS, Wood DE, Aisner DL. et al. Non-small cell lung cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2022; 20 (05) 497-530
- Ettinger DS, Wood DE, Aisner DL. et al. NCCN guidelines insights: non-small cell lung cancer, Version 2.2021. J Natl Compr Canc Netw 2021; 19 (03) 254-266
- Accessed November 15, 2022, at: https://iria.org.in/radiology-imaging-guidelines
- de Groot PM, Chung JH, Ackman JB. et al; Expert Panel on Thoracic Imaging. ACR Appropriateness Criteria® noninvasive clinical staging of primary lung cancer. J Am Coll Radiol 2019; 16 (5S): S184-S195
- Melosky B, Blais N, Cheema P. et al. Standardizing biomarker testing for Canadian patients with advanced lung cancer. Curr Oncol 2018; 25 (01) 73-82
- Schmidt-Hansen M, Baldwin DR, Hasler E, Zamora J, Abraira V, Roqué I Figuls M. PET-CT for assessing mediastinal lymph node involvement in patients with suspected resectable non-small cell lung cancer. Cochrane Database Syst Rev 2014; (11) CD009519 DOI: 10.1002/14651858.CD009519.pub2.
- Brunelli A, Kim AW, Berger KI, Addrizzo-Harris DJ. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143 (5, Suppl): e166S-e190S
- American Cancer Society. Cancer facts & figures 2020. Accessed November 15, 2022, at: https://www.cancer.org/research/cancer-factsstatistics/all-cancer-facts-figures/cancer-facts-figures-2020.html
- Aberle DR, Adams AM, Berg CD. et al; National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365 (05) 395-409
- Henschke CI, McCauley DI, Yankelevitz DF. et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet 1999; 354 (9173): 99-105
- De Koning H, Van Der Aalst C, Ten Haaf K. et al. Effects of volume CT lung cancer screening: mortality results of the NELSON randomized-controlled population-based trial. Paper presented at: 2018 World Conference on Lung Cancer. Abstract PL02.05; 2018
- Kim H, Kim HY, Goo JM, Kim Y. Lung cancer CT screening and lung-RADS in a tuberculosis-endemic country: The Korean Lung Cancer Screening Project (K-LUCAS. Radiology 2020; 296 (01) 181-188
- Yang P. MS16.04 National Lung Screening Program in Taiwan. IASLC 19th World Conf . Lung Cancer 2018; 13 (10, Supplement): S274-S275
- Parang S, Bhavin J. LDCT screening in smokers in India-a pilot, proof-of-concept study. Indian J Radiol Imaging 2021; 31 (02) 318-322
- McKee BJ, Regis SM, McKee AB, Flacke S, Wald C. Performance of ACR lung-RADS in a clinical CT lung screening program. J Am Coll Radiol 2016; 13 (2, Suppl): R25-R29
- Wiener RS, Gould MK, Arenberg DA. et al; ATS/ACCP Committee on Low-Dose CT Lung Cancer Screening in Clinical Practice. An official American Thoracic Society/American College of Chest Physicians policy statement: implementation of low-dose computed tomography lung cancer screening programs in clinical practice. Am J Respir Crit Care Med 2015; 192 (07) 881-891
- Wood DE, Kazerooni EA, Baum SL. et al. Lung Cancer Screening, Version 3.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2018; 16 (04) 412-441
- Kauczor HU, Baird AM, Blum TG. et al; European Society of Radiology (ESR) and the European Respiratory Society (ERS). ESR/ERS statement paper on lung cancer screening. Eur Radiol 2020; 30 (06) 3277-3294
- Nitz JA, Erkmen CP. New 2021 USPSTF lung cancer screening criteria-an opportunity to mitigate racial disparity. JAMA Oncol 2022; 8 (03) 383-384
- Kishi K, Homma S, Kurosaki A. et al. Small lung tumors with the size of 1cm or less in diameter: clinical, radiological, and histopathological characteristics. Lung Cancer 2004; 44 (01) 43-51
- Bueno J, Landeras L, Chung JH. Updated Fleischner Society Guidelines for Managing Incidental Pulmonary Nodules: common questions and challenging scenarios. Radiographics 2018; 38 (05) 1337-1350
- Lacasse Y, Wong E, Guyatt GH, Cook DJ. Transthoracic needle aspiration biopsy for the diagnosis of localised pulmonary lesions: a meta-analysis. Thorax 1999; 54 (10) 884-893
- Silvestri GA, Gonzalez AV, Jantz MA. et al. Methods for staging non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143 (5, Suppl): e211S-e250S
- Birim O, Kappetein AP, Stijnen T, Bogers AJ. Meta-analysis of positron emission tomographic and computed tomographic imaging in detecting mediastinal lymph node metastases in nonsmall cell lung cancer. Ann Thorac Surg 2005; 79 (01) 375-382
- Amin MB. Ed. AJCC Cancer Staging Manual 8th ed. Cham, Switzerland: Springer; 2017: 431-455
- Kalemkerian GP, Gadgeel SM. Modern staging of small cell lung cancer. J Natl Compr Canc Netw 2013; 11 (01) 99-104
- Eisenhauer EA, Therasse P, Bogaerts J. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45 (02) 228-247
- Schwartz LH, Litière S, de Vries E. et al. RECIST 1.1-update and clarification: from the RECIST committee. Eur J Cancer 2016; 62: 132-137
- Seymour L, Bogaerts J, Perrone A. et al; RECIST working group. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 2017; 18 (03) e143-e152
- Strange CD, Shroff GS, Truong MT, Nguyen Q-N, Vlahos I, Erasmus JJ. Imaging of the post-radiation chest in lung cancer. Clin Radiol 2022; 77 (01) 19-30
- Huang K, Senthi S, Palma DA. et al. High-risk CT features for detection of local recurrence after stereotactic ablative radiotherapy for lung cancer. Radiother Oncol 2013; 109 (01) 51-57
- Berghmans T, Dingemans AM, Hendriks LEL, Cadranel J. Immunotherapy for nonsmall cell lung cancer: a new therapeutic algorithm. Eur Respir J 2020; 55 (02) 1901907 DOI: 10.1183/13993003.01907-2019.
- Nishino M, Tirumani SH, Ramaiya NH, Hodi FS. Cancer immunotherapy and immune-related response assessment: the role of radiologists in the new arena of cancer treatment. Eur J Radiol 2015; 84 (07) 1259-1268
- Nishino M, Sholl LM, Hodi FS, Hatabu H, Ramaiya NH. Anti-PD-1-related pneumonitis during cancer immunotherapy. N Engl J Med 2015; 373 (03) 288-290
- Nishino M. Imaging of oncologic treatment-related pneumonitis: a focused review on emerging issues of immune checkpoint inhibitor pneumonitis, from the AJR special series on inflammation. AJR Am J Roentgenol 2022; 218 (01) 19-27
- Nishino M, Ramaiya NH, Awad MM. et al. PD-1 inhibitor-related pneumonitis in advanced cancer patients: radiographic patterns and clinical course. Clin Cancer Res 2016; 22 (24) 6051-6060
- Johkoh T, Lee KS, Nishino M. et al. Chest CT diagnosis and clinical management of drug-related pneumonitis in patients receiving molecular targeting agents and immune checkpoint inhibitors: a position paper from the Fleischner Society. Radiology 2021; 298 (03) 550-566
- Schneider BJ, Ismaila N, Aerts J. et al. Lung cancer surveillance after definitive curative-intent therapy: ASCO guideline. J Clin Oncol 2020; 38 (07) 753-766
- Maconachie R, Mercer T, Navani N, McVeigh G. Guideline Committee. Lung cancer: diagnosis and management: summary of updated NICE guidance. BMJ 2019; 364: l1049 DOI: 10.1136/bmj.l1049.
- Choi JI. Medically inoperable stage I non-small cell lung cancer: best practices and long-term outcomes. Transl Lung Cancer Res 2019; 8 (01) 32-47


 PDF
PDF  Views
Views  Share
Share

