Intracranial Metastases of Intramedullary Spinal Cord Low-Grade Astrocytoma
CC BY-NC-ND 4.0 ? Indian J Med Paediatr Oncol 2020; 41(01): 82-85
DOI: DOI: 10.4103/ijmpo.ijmpo_64_18
Abstract
This paper reports a case of intramedullary spinal cord low-grade (LG) astrocytoma that developed brain metastases after 21 months. A 6-year-old child presented with lower spine pain and falls during daily activity. A spinal cord mass was detected using spinal magnetic resonance imaging (MRI), and brain MRI was normal. The spinal lesion was partially resected, and pathological findings revealed LG astrocytoma (WHO Grade II). The patient underwent thoracolumbar radiotherapy. He returned 21 months following initial admission with symptoms of nausea, vomiting, headaches, and seizure. Brain MRI revealed multiple intracranial masses at the posterior fossa, left lateral ventricle, cerebellopontine angles, and left Meckel cave. A recurrent lesion was detected in the thoracic and lumbar regions of the spinal cord and in the cauda equina. The patient underwent chemotherapy. This rare case warns practitioners to monitor closely the cases of spinal cord astrocytoma that are diagnosed as LG tumors based on histology.
Publication History
Received: 23 March 2018
Accepted: 21 June 2018
Article published online:
23 May 2021
? 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
This paper reports a case of intramedullary spinal cord low-grade (LG) astrocytoma that developed brain metastases after 21 months. A 6-year-old child presented with lower spine pain and falls during daily activity. A spinal cord mass was detected using spinal magnetic resonance imaging (MRI), and brain MRI was normal. The spinal lesion was partially resected, and pathological findings revealed LG astrocytoma (WHO Grade II). The patient underwent thoracolumbar radiotherapy. He returned 21 months following initial admission with symptoms of nausea, vomiting, headaches, and seizure. Brain MRI revealed multiple intracranial masses at the posterior fossa, left lateral ventricle, cerebellopontine angles, and left Meckel cave. A recurrent lesion was detected in the thoracic and lumbar regions of the spinal cord and in the cauda equina. The patient underwent chemotherapy. This rare case warns practitioners to monitor closely the cases of spinal cord astrocytoma that are diagnosed as LG tumors based on histology.
Keywords
Intracranial metastases - low-grade astrocytoma - pediatric - spinal cord tumorIntroduction
Spinal cord astrocytoma is a rare neoplasm in the pediatric population.[1] The previous literature has reported that its prevalence is <1% of all primary neoplasms of the central nervous system[2] [3] [4]?and that it comprises 6%?8% of all primary spinal cord tumors.[5] [6] [7] Brain metastasis of a primary spinal cord astrocytoma has rarely been reported, and most of the reported cases have been caused by high-grade astrocytoma.[8] [9] However, very few cases of intracranial metastasis of low-grade (LG) spinal cord astrocytoma have been reported in pediatrics.[1] Abel?et al. have reported a spinal cord pilocytic astrocytoma that disseminated to the cerebral subarachnoid spaces of a 2-year-old boy.[10] Jang?et al. reported brain metastasis of an intramedullary LG astrocytoma in a 45-year-old patient. This study concluded that the LG spinal cord astrocytoma might spread to intracranial structures without malignant transformation.[4] Ryu?et al. reported two malignant transformations of LG spinal cord astrocytoma among 12 patients.[2] Yamagami?et al. reported a case of a 44-year-old patient with LG astrocytoma that metastasized to the brain after 6 years.[11]
This case report presents intracranial metastasis of LG spinal cord astrocytoma in a child. The purpose of this paper is to provide further evidence for practitioners about the metastasis of initially diagnosed LG spinal cord astrocytoma to the brain specifically in pediatrics.
Case Report
A 6-year-old boy presented with a 1-month history of lower back pain and left crural monoplegia. Spinal magnetic resonance imaging (MRI) revealed an intramedullary spinal cord tumor at the Level T8?T12. Partial resection of the tumor was performed, and the pathological findings of the resected tumor were consistent with LG astrocytoma (WHO grade II). A histological examination of the tumor showed tumoral tissue composed of neoplastic astrocytes with increased cellularity, mild atypia, and low mitotic activity. No vascular endothelial proliferation or necrosis was present [Figure 1].
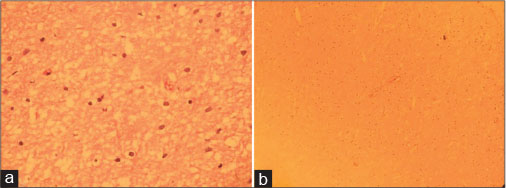
|?Fig. 1: Histological study of the spinal cord tumor (a: H and E, ?400 and b: H and E, ?100) revealed neoplastic astrocytes with slightly pleomorphic enlarged nuclei and no visible cytoplasm, set in a loose fibrillary glial matrix consistent with low-grade astrocytoma
A total dose of 45 Gy was delivered to the spine over 6 weeks. After 2 months, the patient returned with lumbar pain and paraplegia due to a spinal cord abscess [Figure 2]. The spinal cord abscess was removed in the second operation. Paraplegia and urinary/fecal incontinence appeared after surgery. The patient visited as an outpatient several times due to recurrent urinary infections caused by a neurogenic bladder, which occurred before the diagnosis of brain metastasis.
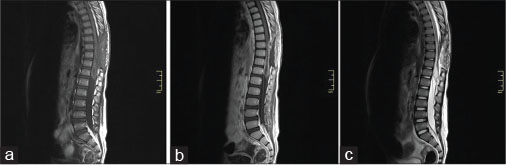
|?Fig. 2: T1-weighted magnetic resonance image with/without contrast (a and b), and T2-weighted magnetic resonance image (c) showing spinal cord abscess appeared following the first operation
Twenty-one months after the first visit, the patient was hospitalized due to seizures and severe headaches. A brain MRI revealed multiple enhancing extra-axial masses at the posterior fossa, left lateral ventricle, cerebellopontine angles, and left Meckel cave, which was compatible with intracranial metastasis [Figure 3]. An elongated, expansile, partially cystic mass was detected in the lower thoracic and lumbar regions of the spinal cord, which extended to the cauda equine [Figure 4]. An assessment for neurofibromatosis Type 1 was negative in this case. The patient underwent the chemotherapy treatment with CCNU, vincristine, and cisplatin. Six months following the diagnosis of cerebral metastases, the patient died due to sepsis caused by the urinary tract infection.
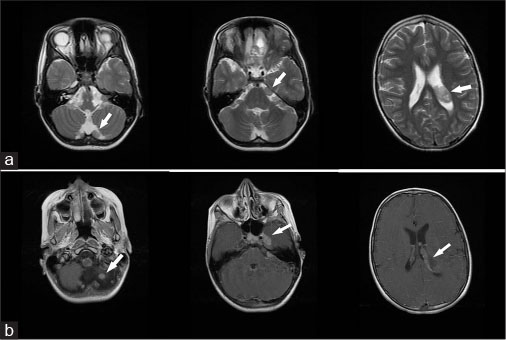
|?Fig. 3: T2-weighted magnetic resonance image (a) and T1-weighted magnetic resonance image with contrast (b) showing multiple extra-axial enhancing masses at the posterior fossa, left lateral ventricle, cerebellopontine angles, and left Meckel cave
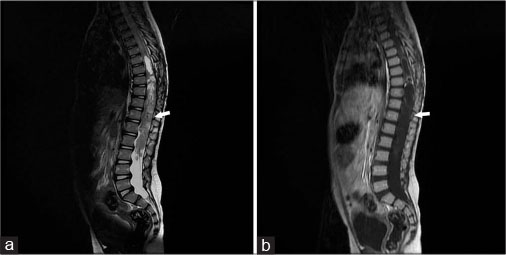
|?Fig. 4: T2-weighted magnetic resonance image (a) and T1-weighted magnetic resonance image with contrast (b) showing an elongated, expansile, partially cystic mass detected in the lower thoracic and lumbar regions of the spinal cord, which extended to the cauda equina
Discussion
This paper reports a case of intracranial metastasis of intramedullary LG astrocytoma in a child. Most cases of intracranial metastasis of spinal cord astrocytoma are related to high-grade tumors and intracranial metastasis of spinal cord LG astrocytoma is a rare phenomenon.[4] [10] [12] The mechanism of intracranial metastasis of intramedullary spinal cord LG astrocytoma is not well known.[10] [13] Some previous researchers have hypothesized that metastasis of LG astrocytoma can develop due to resection and manipulation.[14] However, in a review article, Abel?et al. reported that none of the current studies have proved a correlation between the resection of a LG tumor and its metastasis.[10] Malignant transformation has also been reported as a reason for the brain metastasis of spinal cord LG glioma.[15] [16] However, there is some evidence of brain metastasis of intramedullary LG astrocytoma without malignant transformation.[4] [10] Malignant transformation of LG glioma in children is very unusual compared its occurrence in adults. Irradiation and genetic disorders such as neurofibromatosis-1 were proposed as predisposing factors for malignant transformation of LG glioma.[16] [17] In our patient, radiotherapy had been administered after partial resection of the spinal cord tumor. Intracranial metastasis of the recurrent spinal cord tumor occurred approximately 2 years following the diagnosis of the primary tumor. Malignant transformation is thought to be a possible cause of metastasis in our patient. It was impossible to perform a biopsy on the patient?s recurrent spinal cord tumor due to his condition.
Another hypothesis about our patient is a sampling error in a mixed tumor. In other words, the pathological examination may have been performed on a portion of the primary tumor that contained only the LG tumor and the more invasive part of the tumor was not biopsied. Some studies have reported that histological techniques are probably insufficient to predict the future behavior of a LG glioma. Using biological markers and molecular genetics as parallel techniques is necessary to predict the tumor?s outcomes and behaviors more accurately.[10] [13] Ryu?et al. concluded that although the pathological grade of the tumor is the most important prognostic factor, the biological behavior of the tumor is not always compatible with the pathological findings.[1] [2] A treatment plan should therefore be formulated based on imaging so as to achieve a better therapy.[2] However, there are insufficient radiological criteria to distinguish between benign and malignant tumors based on imaging techniques.
Inconsistent information has been reported on therapy for intramedullary spinal cord astrocytoma, particularly in children.[2] [6] [7] [17] [20] Gross total resection (if possible) is reported as the principal treatment for LG spinal cord astrocytoma. Adjuvant radiation therapy is used for partially resected tumors.[20] [21] [22] However, several studies have reported high levels of morbidity due to more aggressive therapeutic approaches to LG glioma, which have an extreme influence on quality of life.[19] [23] It was the case in our patient, as he had also a high level of postoperative morbidity. Aggressive treatment has therefore only been suggested in specific cases.[19] The role of radiotherapy and chemotherapy for spinal cord astrocytoma is unclear,[22] and these therapies may influence the tumor?s biological behavior and increase the risk of malignant transformation.[16]
This case report warns clinicians regarding the treatment plan of LG spinal cord tumors. The patient should be carefully monitored, and particular attention should be paid to correlating the imaging of the tumor with pathological confirmation.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the legal guardian has given his consent for images and other clinical information to be reported in the journal. The guardian understands that names and initials will not be published and due efforts will be made to conceal patient identity, but anonymity cannot be guaranteed.
Conflict of Interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank Mr. Moahammd Ali Boomeri, for his collaboration with us to report this case.?
References
- Luksik AS, Garzon-Muvdi T, Yang W, Huang J, Jallo GI.?Pediatric spinal cord astrocytomas: A retrospective study of 348 patients from the SEER database. J Neurosurg Pediatr 2017; 19: 711-9
- Ryu SJ, Kim JY, Kim KH, Park JY, Kuh SU, Chin DK. et al.?A retrospective observational study on the treatment outcomes of 26 patients with spinal cord astrocytoma including two cases of malignant transformation. Eur Spine J 2016; 25: 4067-79
- Jeong SM, Chung YG, Lee JB, Shin IY.?Intracranial dissemination from spinal cord anaplastic astrocytoma. J Korean Neurosurg Soc 2010; 47: 68-70
- Jang SY, Kong MH, Song KY, Frazee JG.?Intracranial metastases of cervical intramedullary low-grade astrocytoma without malignant transformation in adult. J Korean Neurosurg Soc 2009; 45: 381-5
- Minehan KJ, Shaw EG, Scheithauer BW, Davis DL, Onofrio BM.?Spinal cord astrocytoma: Pathological and treatment considerations. J Neurosurg 1995; 83: 590-5
- Seki T, Hida K, Yano S, Aoyama T, Koyanagi I, Sasamori T. et al.?Clinical factors for prognosis and treatment guidance of spinal cord astrocytoma. Asian Spine J 2016; 10: 748-54
- Fakhreddine MH, Mahajan A, Penas-Prado M, Weinberg J, McCutcheon IE, Puduvalli V. et al.?Treatment, prognostic factors, and outcomes in spinal cord astrocytomas. Neuro Oncol 2013; 15: 406-12
- Peraud A, Herms J, Schlegel J, M?ller P, Kretzschmar H, Tonn JC. et al.?Recurrent spinal cord astrocytoma with intraventricular seeding. Childs Nerv Syst 2004; 20: 114-8
- Elsamaloty H, Zenooz NA, Mossa-Basha M.?Glioblastoma multiforme (GBM) of the conus medullaris with brain and brain stem metastases. Eur J Radiol Extra 2006; 58: 59-62
- Abel TJ, Chowdhary A, Thapa M, Rutledge JC, Geyer JR, Ojemann J. et al.?Spinal cord pilocytic astrocytoma with leptomeningeal dissemination to the brain. Case report and review of the literature. J Neurosurg 2006; 105: 508-14
- Yamagami T, Kikuchi H, Higashi K, Goto Y, Imataka K.?Intracranial metastasis of a spinal cord astrocytoma ? Case report. Neurol Med Chir (Tokyo) 1990; 30: 69-73
- Gajjar A, Bhargava R, Jenkins JJ, Heideman R, Sanford RA, Langston JW. et al.?Low-grade astrocytoma with neuraxis dissemination at diagnosis. J Neurosurg 1995; 83: 67-71
- Inoue T, Endo T, Nakamura T, Shibahara I, Endo H, Tominaga T. et al.?Expression of CD133 as a putative prognostic biomarker to predict intracranial dissemination of primary spinal cord astrocytoma. World Neurosurg 2018; 110: e715-26
- Bell WO, Packer RJ, Seigel KR, Rorke LB, Sutton LN, Bruce DA. et al.?Leptomeningeal spread of intramedullary spinal cord tumors. Report of three cases. J Neurosurg 1988; 69: 295-300
- Perilongo G, Garr? ML, Giangaspero F.?Low-grade gliomas and leptomeningeal dissemination: A poorly understood phenomenon. Childs Nerv Syst 2003; 19: 197-203
- Winograd E, Pencovich N, Yalon M, Soffer D, Beni-Adani L, Constantini S. et al.?Malignant transformation in pediatric spinal intramedullary tumors: Case-based update. Childs Nerv Syst 2012; 28: 1679-86
- Scheinemann K, Bartels U, Huang A, Hawkins C, Kulkarni AV, Bouffet E. et al.?Survival and functional outcome of childhood spinal cord low-grade gliomas. Clinical article. J Neurosurg Pediatr 2009; 4: 254-61
- Townsend N, Handler M, Fleitz J, Foreman N.?Intramedullary spinal cord astrocytomas in children. Pediatr Blood Cancer 2004; 43: 629-32
- Babu R, Karikari IO, Owens TR, Bagley CA.?Spinal cord astrocytomas: A modern 20-year experience at a single institution. Spine (Phila Pa 1976) 2014; 39: 533-40
- Ahmed R, Menezes AH, Torner JC.?Role of resection and adjuvant therapy in long-term disease outcomes for low-grade pediatric intramedullary spinal cord tumors. J Neurosurg Pediatr 2016; 18: 594-601
- Hsu W, Jallo GI.?Pediatric spinal tumors. In: Dulac O, Lassonde M, Sarnat HB. editors Handbook of Clinical Neurology. Part. 2. Vol. 112. Ch. 100. Amsterdam, The Netherlands: Elsevier; 2013: 959-65
- Newton HB.?Overview of pathology and treatment of primary spinal cord tumors. In: Handbook of Neuro-Oncology Neuroimaging. 2nd ed. Ch. 5 San Diego: Academic Press; 2016: 41-53
- Xiao R, Miller JA, Abdullah KG, Lubelski D, Mroz TE, Benzel EC. et al.?Quality of life outcomes following resection of adult intramedullary spinal cord tumors. Neurosurgery 2016; 78: 821-8
Address for correspondence
Publication History
Received: 23 March 2018
Accepted: 21 June 2018
Article published online:
23 May 2021
? 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

|?Fig. 1: Histological study of the spinal cord tumor (a: H and E, ?400 and b: H and E, ?100) revealed neoplastic astrocytes with slightly pleomorphic enlarged nuclei and no visible cytoplasm, set in a loose fibrillary glial matrix consistent with low-grade astrocytoma

|?Fig. 2: T1-weighted magnetic resonance image with/without contrast (a and b), and T2-weighted magnetic resonance image (c) showing spinal cord abscess appeared following the first operation

|?Fig. 3: T2-weighted magnetic resonance image (a) and T1-weighted magnetic resonance image with contrast (b) showing multiple extra-axial enhancing masses at the posterior fossa, left lateral ventricle, cerebellopontine angles, and left Meckel cave

|?Fig. 4: T2-weighted magnetic resonance image (a) and T1-weighted magnetic resonance image with contrast (b) showing an elongated, expansile, partially cystic mass detected in the lower thoracic and lumbar regions of the spinal cord, which extended to the cauda equina
References
- 1?Luksik AS, Garzon-Muvdi T, Yang W, Huang J, Jallo GI.?Pediatric spinal cord astrocytomas: A retrospective study of 348 patients from the SEER database. J Neurosurg Pediatr 2017; 19: 711-9
- 2?Ryu SJ, Kim JY, Kim KH, Park JY, Kuh SU, Chin DK. et al.?A retrospective observational study on the treatment outcomes of 26 patients with spinal cord astrocytoma including two cases of malignant transformation. Eur Spine J 2016; 25: 4067-79
- 3?Jeong SM, Chung YG, Lee JB, Shin IY.?Intracranial dissemination from spinal cord anaplastic astrocytoma. J Korean Neurosurg Soc 2010; 47: 68-70
- 4?Jang SY, Kong MH, Song KY, Frazee JG.?Intracranial metastases of cervical intramedullary low-grade astrocytoma without malignant transformation in adult. J Korean Neurosurg Soc 2009; 45: 381-5
- 5?Minehan KJ, Shaw EG, Scheithauer BW, Davis DL, Onofrio BM.?Spinal cord astrocytoma: Pathological and treatment considerations. J Neurosurg 1995; 83: 590-5
- 6?Seki T, Hida K, Yano S, Aoyama T, Koyanagi I, Sasamori T. et al.?Clinical factors for prognosis and treatment guidance of spinal cord astrocytoma. Asian Spine J 2016; 10: 748-54
- 7?Fakhreddine MH, Mahajan A, Penas-Prado M, Weinberg J, McCutcheon IE, Puduvalli V. et al.?Treatment, prognostic factors, and outcomes in spinal cord astrocytomas. Neuro Oncol 2013; 15: 406-12
- 8?Peraud A, Herms J, Schlegel J, M?ller P, Kretzschmar H, Tonn JC. et al.?Recurrent spinal cord astrocytoma with intraventricular seeding. Childs Nerv Syst 2004; 20: 114-8
- 9?Elsamaloty H, Zenooz NA, Mossa-Basha M.?Glioblastoma multiforme (GBM) of the conus medullaris with brain and brain stem metastases. Eur J Radiol Extra 2006; 58: 59-62
- 10?Abel TJ, Chowdhary A, Thapa M, Rutledge JC, Geyer JR, Ojemann J. et al.?Spinal cord pilocytic astrocytoma with leptomeningeal dissemination to the brain. Case report and review of the literature. J Neurosurg 2006; 105: 508-14
- 11?Yamagami T, Kikuchi H, Higashi K, Goto Y, Imataka K.?Intracranial metastasis of a spinal cord astrocytoma ? Case report. Neurol Med Chir (Tokyo) 1990; 30: 69-73
- 12?Gajjar A, Bhargava R, Jenkins JJ, Heideman R, Sanford RA, Langston JW. et al.?Low-grade astrocytoma with neuraxis dissemination at diagnosis. J Neurosurg 1995; 83: 67-71
- 13?Inoue T, Endo T, Nakamura T, Shibahara I, Endo H, Tominaga T. et al.?Expression of CD133 as a putative prognostic biomarker to predict intracranial dissemination of primary spinal cord astrocytoma. World Neurosurg 2018; 110: e715-26
- 14?Bell WO, Packer RJ, Seigel KR, Rorke LB, Sutton LN, Bruce DA. et al.?Leptomeningeal spread of intramedullary spinal cord tumors. Report of three cases. J Neurosurg 1988; 69: 295-300
- 15?Perilongo G, Garr? ML, Giangaspero F.?Low-grade gliomas and leptomeningeal dissemination: A poorly understood phenomenon. Childs Nerv Syst 2003; 19: 197-203
- 16?Winograd E, Pencovich N, Yalon M, Soffer D, Beni-Adani L, Constantini S. et al.?Malignant transformation in pediatric spinal intramedullary tumors: Case-based update. Childs Nerv Syst 2012; 28: 1679-86
- 17?Scheinemann K, Bartels U, Huang A, Hawkins C, Kulkarni AV, Bouffet E. et al.?Survival and functional outcome of childhood spinal cord low-grade gliomas. Clinical article. J Neurosurg Pediatr 2009; 4: 254-61
- 18?Townsend N, Handler M, Fleitz J, Foreman N.?Intramedullary spinal cord astrocytomas in children. Pediatr Blood Cancer 2004; 43: 629-32
- 19?Babu R, Karikari IO, Owens TR, Bagley CA.?Spinal cord astrocytomas: A modern 20-year experience at a single institution. Spine (Phila Pa 1976) 2014; 39: 533-40
- 20?Ahmed R, Menezes AH, Torner JC.?Role of resection and adjuvant therapy in long-term disease outcomes for low-grade pediatric intramedullary spinal cord tumors. J Neurosurg Pediatr 2016; 18: 594-601
- 21?Hsu W, Jallo GI.?Pediatric spinal tumors. In: Dulac O, Lassonde M, Sarnat HB. editors Handbook of Clinical Neurology. Part. 2. Vol. 112. Ch. 100. Amsterdam, The Netherlands: Elsevier; 2013: 959-65
- 22?Newton HB.?Overview of pathology and treatment of primary spinal cord tumors. In: Handbook of Neuro-Oncology Neuroimaging. 2nd ed. Ch. 5 San Diego: Academic Press; 2016: 41-53
- 23?Xiao R, Miller JA, Abdullah KG, Lubelski D, Mroz TE, Benzel EC. et al.?Quality of life outcomes following resection of adult intramedullary spinal cord tumors. Neurosurgery 2016; 78: 821-8


 PDF
PDF  Views
Views  Share
Share

