Metabolic Syndrome and Breast Cancer Risk
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(04): 434-439
DOI: DOI: 10.4103/ijmpo.ijmpo_168_16
Abstract
Objective: The study was meant to estimate the prevalence of metabolic syndrome in patients with breast cancer and to establish its role as an independent risk factor on occurrence of breast cancer. Materials and Methods: Fifty women aged between 40 and 80 years with breast cancer and fifty controls of similar age were assessed for metabolic syndrome prevalence and breast cancer risk factors, including age at menarche, reproductive status, live births, breastfeeding, and family history of breast cancer, age at diagnosis of breast cancer, body mass index, and metabolic syndrome parameters. Results: Metabolic syndrome prevalence was found in 40.0% of breast cancer patients, and 18.0% of those in control group (P = 0.02). An independent and positive association was seen between metabolic syndrome and breast cancer risk (odds ratio = 3.037; 95% confidence interval 1.214–7.597). Conclusions: Metabolic syndrome is more prevalent in breast cancer patients and is an independent risk factor for breast cancer.
Publication History
Article published online:
04 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used forcommercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Objective:
The study was meant to estimate the prevalence of metabolic syndrome in patients with breast cancer and to establish its role as an independent risk factor on occurrence of breast cancer.
Materials and Methods:
Fifty women aged between 40 and 80 years with breast cancer and fifty controls of similar age were assessed for metabolic syndrome prevalence and breast cancer risk factors, including age at menarche, reproductive status, live births, breastfeeding, and family history of breast cancer, age at diagnosis of breast cancer, body mass index, and metabolic syndrome parameters.
Results:
Metabolic syndrome prevalence was found in 40.0% of breast cancer patients, and 18.0% of those in control group (P = 0.02). An independent and positive association was seen between metabolic syndrome and breast cancer risk (odds ratio = 3.037; 95% confidence interval 1.214–7.597).
Conclusions:
Metabolic syndrome is more prevalent in breast cancer patients and is an independent risk factor for breast cancer.
Introduction
Metabolic syndrome (MS), variously called as insulin resistance syndrome or syndrome X or Reaven syndrome, consists of a constellation of metabolic abnormalities which include central obesity, hyperglycemia, hyperinsulinemia, hypertension, hypertriglyceridemia, low high-density lipoprotein (HDL) cholesterol, hyperuricemia, and increased levels of fibrinogen,[1] that confer increased risk of cardiovascular disease and diabetes mellitus.[2,3] MS has recently been proposed to play a part in breast carcinogenesis.[4] The metabolic syndrome could impact the risk of breast cancer through alterations in a number of interrelated hormonal pathways, including those involving insulin, estrogen, cytokines, and growth factors.[4,5]
Clinical and investigational evidence suggests that the increased breast cancer risk associated with greater abdominal visceral obesity may be related to anomalous insulin signaling through the insulin receptor substrate 1 pathway, leading to insulin resistance, hyperinsulinemia, and increased concentrations of endogenous estrogen and androgen.[6]
So far, many studies have analyzed the association of individual MS components with breast cancer risk, but only a few studied the role of MS per se in relation to breast cancer occurrence.[7,8,9,10,11,12,13,14,15,16,17,18,19] The present study was aimed to estimate the prevalence of metabolic syndrome in patients with breast cancer and to establish its role as an independent risk factor on occurrence of breast cancer.
Materials and Methods
The study was undertaken in the Departments of Medical Oncology, Endocrinology and Clinical Biochemistry, Sher-i-Kashmir Institute of Medical Sciences, Soura. The study was approved by Ethical Committee SKIMS. The study was conducted from October 2012 to September 2014.
Subjects
Patients affected by histologically confirmed breast cancer either pre- or post-menopausal were recruited for the study. An informed consent was taken from both individuals and their family members after detailed explanation of the procedure to them. Those who give informed consent were taken for the study.
Controls
Around 50 apparently healthy women both pre- and post-menopausal were taken as controls.
Exclusion criteria
The individuals who had any history of systemic disease such as known diabetes, heart diseases, current pregnancy, lactation, history of drug intake such as steroids, androgens, oral contraceptives, drugs known to interfere in glucose, or lipid metabolism were excluded from the study.
Methods
Each of these participants was interviewed about:
- Interview data
- Detailed menstrual history including age of menarche, regularity, duration, and number of menstrual cycles per year
- Detailed obstetric history including any infertility, drugs used for infertility, number of conceptions, any abortions, duration of lactation, and use of oral contraceptives
- Personal history of metabolic and cardiovascular disease features of acne, any hair loss, or abnormal hair growth
- Family history of metabolic and neoplastic diseases.
Physical examination
All females were subjected to assessment of anthropometric parameters such as measurement of body weight, height, and the waist and hip circumferences. Body weight was measured by electronic scale (Filizola) to the nearest 0.1 kg while individuals were barefoot and wearing light clothes. Height was determined by portable Seca stadiometer to nearest 01.0 cm, according to norms proposed by World Health Organization (1995). Measured weight and height were used to calculate the body mass index (BMI, weight in kilograms divided by height in meters squared). The smallest waist circumference and the largest hip circumference were used to calculate waist to hip circumference ratio (WHR).
Obesity was defined as BMI value of 27 kg/m2 or larger.
Upper body fat distribution was defined as WHR >0.8.
Blood pressure (BP) was measured on the right arm with individuals lying to standing by means of mercury sphygmomanometer after the individual had been at rest for a minimum period of 5 minutes and the cuff involved 80% of the right arm's circumference. The point of disappearance of Korotkoff sounds (Phase V) was recorded as patient's diastolic BP. The average of three measurements will be taken as the individuals' BP value. Hypertension was defined by Seventh Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure Criteria.
Investigations
A sample of about 10 ml blood was taken from each female after overnight fasting ≥ 8h. The samples were sent for the following tests:
- Fasting plasma glucose
- Serum triglyceride levels
- HDL levels
- Low-density lipoprotein levels
- Serum cholesterol levels.
Assays
- Glucose was measured by the oxidize glucose method
- Lipid profile, KFT was done by HITACHE.
These investigations were carried out at SKIMS Bio technology laboratory. Impaired glucose metabolism and type 2 Diabetes Mellitus were defined by American Diabetes Association criteria. Impaired fasting glucose was defined by fasting blood glucose levels = 100 mg/dl but <126>
Definition of the metabolic syndrome
The National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) criteria will be employed for diagnosis of MS as per following criteria:
Presence of three or more of the following clinical criteria:
- Waist circumference >88 cm
- BP ≥135/85 mmHg
- HDL cholesterol <50>
- Triglyceride level ≥150 mg/dl
- Fasting plasma glucose ≥100 mg/dl.
Statistical analysis
Entire data were subjected to suitable standard statistical technique. Univariate analysis was done applying Chi-square test, t-test. The analyses were performed using SPSS statistical package 20 (SPSS Inc., IBM, Chicago, IL, USA).
Results and Observations
Table 1 shows the main results for the metabolic syndrome and its components. There was no statistically significant difference in number of individuals in various age groups between cases (mean age 54.28 ± 7.36 years) and controls (50.96 ± 9.94 years). Age, number of live births, and menopausal status did not seem to affect breast cancer risk. The prevalence of obesity was similar in both case and control groups. Significant difference was found considering the prevalence of upper body fat distribution. Family history of breast cancer was more frequent in cases than in controls (P < 0 xss=removed>P = 0.02). Metabolic syndrome was strongly associated with breast cancer risk. Relative to the control group, patients with metabolic syndrome were more than three times more likely to have breast cancer (odds ratio (OR) = 3.037; 95% confidence interval (CI) 1.214–7.597). Moreover, some of the individual components of the syndrome were associated with increased breast cancer risk, including higher waist circumference (OR = 4.992; 95% CI 2.132–11.685), BP levels (OR = 6.000; 95% CI 2.031–17.728) and serum triglyceride levels (OR = 4.929; 95% CI 1.503–16.157) and fasting glucose levels (OR = 6.714; 95% CI 1.803–24.998). However, low HDL levels did not seem to affect breast cancer risk as it did not achieve statistical significance between case and control groups [Table 1].
Table 1
Metabolic syndrome components
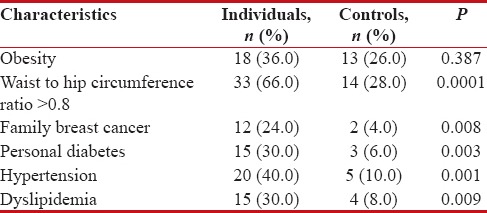
|
Discussion
Metabolic syndrome consists of constellations of metabolic abnormalities including obesity, type 2 diabetes mellitus, hypertension, dyslipidemia, and is characterized physiopathologically by the common feature of hyperinsulinemia/insulin resistance.[1] Breast cancer risk is postulated to be driven by cell proliferation in response to sex hormones but also to polypeptide growth hormones (GHs) (insulin-like growth factors (IGFs) and insulin itself).[20,21,22,23] Studies suggest that hyperinsulinemia and its sequelae can increase the promotion of breast carcinogenesis, and the mechanism is likely related to increased bioactivity of IGF-1. In the present study, metabolic syndrome was identified by blood determinations to ensure the quality of metabolic syndrome diagnosis, toward estimating metabolic syndrome as a risk factor for breast cancer that possibly increases the risk prediction beyond what may already seem apparent by metabolic syndrome components. The present study reflected important finding of the increased prevalence of hypertension, personal type 2 diabetes mellitus, and dyslipidemia in individuals with documented breast cancer versus controls. Our observation exhibited higher prevalence of MS (40%) among women with breast cancer in postmenopausal compared to healthy women (18%).
Metabolic syndrome is an insulin resistance syndrome, and several studies have implicated insulin in breast cancer development.[24] C-peptide serum levels – indicator of pancreatic insulin production – is linked with increased breast cancer risk in postmenopausal women,[5] and in breast cancer patients, high serum insulin is associated with poorer outcome.[25] Insulin has a gonadotropic effect.[26] It promotes the ovarian stroma to produce androgens, whose peripheral aromatization is the main source of estrogens after menopause.[27,28] Insulin also augments aromatase activity.[29] Abdominal adipose, in particular, is an important source of both androgens and estrogens.[30] Obese women have high levels of estrogens, which are widely considered to facilitate the association of obesity with breast cancer risk.[31] In the present study, however, obesity was not associated with breast cancer risk, suggesting that metabolic syndrome has an effect that is independent of obesity.
Insulin also reduces liver production of sex hormone-binding globulin (SHBG), thereby increasing sex hormone bioavailability,[32] and metabolic syndrome is associated with increased levels of both total and free[33] testosterone.
A supplementary mechanism by which insulin may escalate breast cancer risk is through its effect on the bioavailability of IGF-I. Insulin declines hepatic production of two IGF-binding proteins, IGFBP1 and IGFBP2,[34,35] thereby increasing IGF-I bioavailability and enhances the synthesis of GH-receptor,[36,37] thus allowing GH to promote IGF-I synthesis. Both insulin[38] and IGF-I conjoin with estrogens to stimulate the proliferation of breast epithelium cells.[39] Many studies have examined the relationship of breast cancer with prediagnostic serum levels of IGF-I, with inconsistent results.[39] The initial studies found a positive association only in premenopausal women;[31,40,41] more recent studies on larger cohorts, however, did not endorse an association before menopause but emphasized a significant positive association after menopause.[42,43]
Metabolic syndrome is also associated with increasing levels of inflammatory cytokines[44] and leptin,[45] which can stimulate cell proliferation through various mechanisms,[46,47] and is inversely associated with adiponectin,[48] which downregulates tumor cell proliferation and upregulates apoptosis.[47]
Several studies have revealed that waist circumference is a significant predictor of breast cancer risk in postmenopausal women, and the positive link between abdominal obesity and breast cancer was found to be independent of BMI.[9,10] In the present study, abdominal obesity was associated with increased breast cancer risk. As the development of metabolic syndrome has been associated to obesity and physical inactivity, it is important to encourage increased physical activity and healthy dietary practices to diminish the prevalence of obesity in the population and the probable relationship between postmenopausal breast cancer and physical inactivity. Weight reduction combined with regular physical exercise has been displayed to decrease both estrogen and insulin concentrations in obese women, and such a regimen might be investigated in clinical trials for an effect on breast cancer risk in obese women.
Hypertension, which occurs as a single entity or as part of the metabolic syndrome, has been associated to be a risk factor for breast cancer.[49,50,51] The mechanisms underlying this association remain unclear, but breast cancer and hypertension may have common pathophysiologic means, including those involved in subclinical inflammation. In the present study, hypertension had an adverse effect on breast cancer risk although BP could be elevated in the affected individuals purely because of the anxiety of attending an oncology consultation appointment.
Low serum HDL cholesterol has lately been associated with increased risk of breast cancer in overweight and obese women.[52] In the present study, however, HDL cholesterol did not seem to affect breast cancer risk. Elevated levels of plasma triglycerides are associated with increased risk of histologically documented premenopausal breast cancer. In this study, elevated serum triglyceride levels were found to be significantly associated with breast cancer risk. This association persevered after adjustment for age, body size, lipids, reproductive and familial risk factors, in keeping with an independent association of elevated triglycerides with breast cancer risk.
One exciting findings of the present study was the association of fasting glucose with breast cancer risk in both premenopausal and postmenopausal women. Schoen et al.[53] reported that fasting glucose was associated with colorectal cancer, another type of cancer of which the etiology has been related to impaired fasting glucose and hyperinsulinemic insulin resistance. Another prospective study, the Malmo Diet and Cancer Study, did not find an association between fasting glucose and breast cancer risk in premenopausal or postmenopausal women.[16] Fasting glucose levels, after an overnight fast, depend on the hepatic and renal gluconeogenesis.[54] Apart from decrease in insulin sensitivity or insulin secretion, which cause increased glucose production and decreased glucose utilization,[55] gluconeogenesis is stimulated by counterregulatory hormones such as adrenal hormones, androgens, and GHs.[56,57] These hormones are determinants of fasting glucose, and additional studies are needed to elucidate the potential etiological role of these hormones in breast cancer. On the other hand, one can hypothesize that increased serum glucose availability may offer a selective advantage to malignant cells with increased serum glucose requirement.[58] In addition, glucose itself may promote carcinogenic processes through the production of free radicals and the induction of oxidative injury to both DNA and to the enzymes concerned with the repair and processing of DNA.[59,60,61,62]
Limitations
There are important basic limitations of case–control studies. We tried to attenuate the effects of confounding factors by matching cases and controls for age, by enrolling cases and controls at the same time of the year and from the same institution. However, the controls seem to be younger than the affected patients. Although this is not statistically significant, it raises the question as to whether or not the age distribution of affected patients compared with controls could affect the overall results.
Conclusions
Our study adds evidence to the little understanding existing on the association of metabolic syndrome with breast cancer risk, confirming that metabolic syndrome is associated with breast cancer. Furthermore, the prevalence of metabolic syndrome is high and increasing with an increasing incidence of breast cancer worldwide. Multimodality actions involving nutrition, exercise, and stress reduction may be extremely important to control insulin resistance and improve cancer outcomes. Interventions to cut breast cancer risk in first-degree relatives of breast cancer patients need to be initiated at an early age.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and itscomplications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998;15:539-53.
- Sathyaprakash R, Henry RR. Preventing diabetes by treating aspects of the metabolic syndrome. Curr Diab Rep 2002;2:416-22.
- Alexander CM, Landsman PB, Teutsch SM, Haffner SM, Third National Health and Nutrition Examination Survey (NHANES III), National Cholesterol Education Program (NCEP), et al. NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes 2003;52:1210-4.
- Xue F, Michels KB. Diabetes, metabolic syndrome, and breast cancer: A review of the current evidence. Am J Clin Nutr 2007;86:s823-35.
- Vona-Davis L, Howard-McNatt M, Rose DP. Adiposity, type 2 diabetes and the metabolic syndrome in breast cancer. Obes Rev 2007;8:395-408.
- Stoll BA, Secreto G. New hormone-related markers of high risk to breast cancer. Ann Oncol 1992;3:435-8.
- Folsom AR, Kaye SA, Prineas RJ, Potter JD, Gapstur SM, Wallace RB, et al. Increased incidence of carcinoma of the breast associated with abdominal adiposity in postmenopausal women. Am J Epidemiol 1990;131:794-803.
- Huang Z, Willett WC, Colditz GA, Hunter DJ, Manson JE, Rosner B, et al. Waist circumference, waist: Hip ratio, and risk of breast cancer in the nurses' health study. Am J Epidemiol 1999;150:1316-24.
- den Tonkelaar I, Seidell JC, Collette HJ. Body fat distribution in relation to breast cancer in women participating in the DOM-project. Breast Cancer Res Treat 1995;34:55-61.
- ;Sonnenschein E, Toniolo P, Terry MB, Bruning PF, Kato I, Koenig KL, et al. Body fat distribution and obesity in pre- and postmenopausal breast cancer. Int J Epidemiol 1999;28:1026-31.
- Bani IA, Williams CM, Boulter PS, Dickerson JW. Plasma lipids and prolactin in patients with breast cancer. Br J Cancer 1986;54:439-46.
- Schreier LE, Berg GA, Basilio FM, Lopez GI, Etkin AE, Wikinski RL, et al. Lipoprotein alterations, abdominal fat distribution and breast cancer. Biochem Mol Biol Int 1999;47:681-90.
- Høyer AP, Engholm G. Serum lipids and breast cancer risk: A cohort study of 5,207 Danish women. Cancer Causes Control 1992;3:403-8.
- ;Gaard M, Tretli S, Urdal P. Risk of breast cancer in relation to blood lipids: A prospective study of 31,209 Norwegian women. Cancer Causes Control 1994;5:501-9.
- Törnberg SA, Holm LE, Carstensen JM. Breast cancer risk in relation to serum cholesterol, serum beta-lipoprotein, height, weight, and blood pressure. Acta Oncol 1988;27:31-7.
- Manjer J, Kaaks R, Riboli E, Berglund G. Risk of breast cancer in relation to anthropometry, blood pressure, blood lipids and glucose metabolism: A prospective study within the malmö preventive project. Eur J Cancer Prev 2001;10:33-42.
- Muti P, Quattrin T, Grant BJ, Krogh V, Micheli A, Schünemann HJ, et al. Fasting glucose is a risk factor for breast cancer: A prospective study. Cancer Epidemiol Biomarkers Prev 2002;11:1361-8.
- Mink PJ, Shahar E, Rosamond WD, Alberg AJ, Folsom AR. Serum insulin and glucose levels and breast cancer incidence: The atherosclerosis risk incommunities study. Am J Epidemiol 2002;156:349-52.
- Lawlor DA, Smith GD, Ebrahim S. Hyperinsulinaemia and increased risk of breast cancer: Findings from the British women's heart and health study. Cancer Causes Control 2004;15:267-75.
- Benson EA, Holdaway IM. Regulation of insulin binding to human mammary carcinoma. Cancer Res 1982;42:1137-41.
- ;Milazzo G, Giorgino F, Damante G, Sung C, Stampfer MR, Vigneri R, et al. Insulin receptor expression and function in human breast cancer cell lines. Cancer Res 1992;52:3924-30.
- Cullen KJ, Yee D, Sly WS, Perdue J, Hampton B, Lippman ME, et al. Insulin-like growth factor receptor expression and function in human breast cancer. Cancer Res 1990;50:48-53.
- Reddy KB, Mangold GL, Tandon AK, Yoneda T, Mundy GR, Zilberstein A, et al. Inhibition of breast cancer cell growth in vitro by a tyrosine kinase inhibitor. Cancer Res 1992;52:3636-41.
- Kaaks R. Nutrition, hormones, and breast cancer: Is insulin the missing link? Cancer Causes Control 1996;7:605-25.
- Goodwin PJ, Ennis M, Pritchard KI, Trudeau ME, Koo J, Madarnas Y, et al. Fasting insulin and outcome in early-stage breast cancer: Results of a prospective cohort study. J Clin Oncol 2002;20:42-51.
- Poretsky L, Cataldo NA, Rosenwaks Z, Giudice LC. The insulin-related ovarian regulatory system in health and disease. Endocr Rev 1999;20:535-82.
- Berrino F, Muti P, Micheli A, Bolelli G, Krogh V, Sciajno R, et al. Serum sex hormone levels after menopause and subsequent breast cancer. J Natl Cancer Inst 1996;88:291-6.
- Nelson LR, Bulun SE. Estrogen production and action. J Am Acad Dermatol 2001;45:S116-24.
- McTernan PG, Anwar A, Eggo MC, Barnett AH, Stewart PM, Kumar S, et al. Gender differences in the regulation of P450 aromatase expression and activity in human adipose tissue. Int J Obes Relat Metab Disord 2000;24:875-81.
- Cauley JA, Gutai JP, Kuller LH, LeDonne D, Powell JG. The epidemiology of serum sex hormones in postmenopausal women. Am J Epidemiol 1989;129:1120-31.
- Key TJ, Appleby PN, Reeves GK, Roddam A, Dorgan JF, Longcope C, et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst 2003;95:1218-26.
- Sherif K, Kushner H, Falkner BE. Sex hormone-binding globulin and insulin resistance in African-American women. Metabolism 1998;47:70-4.
- Golden SH, Ding J, Szklo M, Schmidt MI, Duncan BB, Dobs A, et al. Glucose and insulincomponents of the metabolic syndrome are associated with hyperandrogenism in postmenopausal women: The atherosclerosis risk incommunities study. Am J Epidemiol 2004;160:540-8.
- Brismar K, Hilding A, Lindgren B. Regulation of IGFBP-1 in humans. Prog Growth Factor Res 1995;6:449-56.
- Norat T, Dossus L, Rinaldi S, Overvad K, Grønbaek H, Tjønneland A, et al. Diet, serum insulin-like growth factor-I and IGF-binding protein-3 in European women. Eur J Clin Nutr 2007;61:91-8.
- Hanaire-Broutin H, Sallerin-Caute B, Poncet MF, Tauber M, Bastide R, Rosenfeld R, et al. Insulin therapy and GH-IGF-I axis disorders in diabetes: Impact of glycaemic control and hepatic insulinization. Diabetes Metab 1996;22:245-50.
- Tollet P, Enberg B, Mode A. Growth hormone (GH) regulation of cytochrome P-450IIC12, insulin-like growth factor-I (IGF-I), and GH receptor messenger RNA expression in primary rat hepatocytes: A hormonal interplay with insulin, IGF-I, and thyroid hormone. Mol Endocrinol 1990;4:1934-42.
- Panno ML, Salerno M, Pezzi V, Sisci D, Maggiolini M, Mauro L, et al. Effect of oestradiol and insulin on the proliferative pattern and on oestrogen and progesterone receptor contents in MCF-7 cells. J Cancer Res Clin Oncol 1996;122:745-9.
- Campagnoli C, Pasanisi P, Peris C, Berrino F. Insulin-like growth factor-I and breast cancer: Epidemiological and clinical data. Breast Cancer: Prognosis, Treatment, and Prevention. In: Jorge R. Pasqualini, editors. CRC Press 2008. p. 323-42.
- Hankinson SE, Willett WC, Colditz GA, Hunter DJ, Michaud DS, Deroo B, et al. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet 1998;351:1393-6.
- Toniolo P, Bruning PF, Akhmedkhanov A, Bonfrer JM, Koenig KL, Lukanova A, et al. Serum insulin-like growth factor-I and breast cancer. Int J Cancer 2000;88:828-32.
- Baglietto L, English DR, Hopper JL, Morris HA, Tilley WD, Giles GG, et al. Circulating insulin-like growth factor-I and binding protein-3 and the risk of breast cancer. Cancer Epidemiol Biomarkers Prev 2007;16:763-8.
- Rinaldi S, Peeters PH, Berrino F, Dossus L, Biessy C, Olsen A, et al. IGF-I, IGFBP-3 and breast cancer risk in women: The European prospective investigation into cancer and nutrition (EPIC). Endocr Relat Cancer 2006;13:593-605.
- Rose DP, Haffner SM, Baillargeon J. Adiposity, the metabolic syndrome, and breast cancer in African-American and white American women. Endocr Rev 2007;28:763-77.
- Siemińska L, Wojciechowska C, Foltyn W, Kajdaniuk D, Kos-Kudła B, Marek B, et al. The relation of serum adiponectin and leptin levels to metabolic syndrome in women before and after the menopause. Endokrynol Pol 2006;57:15-22.
- Purohit A, Newman SP, Reed MJ. The role of cytokines in regulating estrogen synthesis: Implications for the etiology of breast cancer. Breast Cancer Res 2002;4:65-9.
- Vona-Davis L, Rose DP. Adipokines as endocrine, paracrine, and autocrine factors in breast cancer risk and progression. Endocr Relat Cancer 2007;14:189-206.
- Ryo M, Nakamura T, Kihara S, Kumada M, Shibazaki S, Takahashi M, et al. Adiponectin as a biomarker of the metabolic syndrome. Circ J 2004;68:975-81.
- Franceschi S, la Vecchia C, Negri E, Parazzini F, Boyle P. Breast cancer risk and history of selected medical conditions linked with female hormones. Eur J Cancer 1990;26:781-5.
- de Waard F, Baanders-van Halewijn EA. A prospective study in general practice on breast-cancer risk in postmenopausal women. Int J Cancer 1974;14:153-60.
- Moseson M, Koenig KL, Shore RE, Pasternack BS. The influence of medical conditions associated with hormones on the risk of breast cancer. Int J Epidemiol 1993;22:1000-9.
- Furberg AS, Jasienska G, Bjurstam N, Torjesen PA, Emaus A, Lipson SF, et al. Metabolic and hormonal profiles: HDL cholesterol as a plausible biomarker of breast cancer risk. The Norwegian EBBA study. Cancer Epidemiol Biomarkers Prev 2005;14:33-40.
- Schoen RE, Tangen CM, Kuller LH, Burke GL, Cushman M, Tracy RP, et al. Increased blood glucose and insulin, body size, and incident colorectal cancer. J Natl Cancer Inst 1999;91:1147-54.
- Rothman DL, Magnusson I, Katz LD, Shulman RG, Shulman GI. Quantitation of hepatic glycogenolysis and gluconeogenesis in fasting humans with 13C NMR. Science 1991;254:573-6.
- Rizza RA, Mandarino LJ, Gerich JE. Dose-response characteristics for effects of insulin on production and utilization of glucose in man. Am J Physiol 1981;240:E630-9.
- Deibert DC, DeFronzo RA. Epinephrine-induced insulin resistance in man. J Clin Invest 1980;65:717-21.
- MacGorman LR, Rizza RA, Gerich JE. Physiological concentrations of growth hormone exert insulin-like and insulin antagonistic effects on both hepatic and extrahepatic tissues in man. J Clin Endocrinol Metab 1981;53:556-9.
- Sun Y. Free radicals, antioxidant enzymes, and carcinogenesis. Free Radic Biol Med 1990;8:583-99.
- Sipe HJ Jr., Jordan SJ, Hanna PM, Mason RP. The metabolism of 17 beta-estradiol by lactoperoxidase: A possible source of oxidative stress in breast cancer. Carcinogenesis 1994;15:2637-43.
- Dandona P, Thusu K, Cook S, Snyder B, Makowski J, Armstrong D, et al. Oxidative damage to DNA in diabetes mellitus. Lancet 1996;347:444-5.
- Kitahara M, Eyre HJ, Lynch RE, Rallison ML, Hill HR. Metabolic activity of diabetic monocytes. Diabetes 1980;29:251-6.
- Armstrong D, al-Awadi F. Lipid peroxidation and retinopathy in streptozotocin-induced diabetes. Free Radic Biol Med 1991;11:433-6.
References
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and itscomplications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998;15:539-53.
- Sathyaprakash R, Henry RR. Preventing diabetes by treating aspects of the metabolic syndrome. Curr Diab Rep 2002;2:416-22.
- Alexander CM, Landsman PB, Teutsch SM, Haffner SM, Third National Health and Nutrition Examination Survey (NHANES III), National Cholesterol Education Program (NCEP), et al. NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes 2003;52:1210-4.
- Xue F, Michels KB. Diabetes, metabolic syndrome, and breast cancer: A review of the current evidence. Am J Clin Nutr 2007;86:s823-35.
- Vona-Davis L, Howard-McNatt M, Rose DP. Adiposity, type 2 diabetes and the metabolic syndrome in breast cancer. Obes Rev 2007;8:395-408.
- Stoll BA, Secreto G. New hormone-related markers of high risk to breast cancer. Ann Oncol 1992;3:435-8.
- Folsom AR, Kaye SA, Prineas RJ, Potter JD, Gapstur SM, Wallace RB, et al. Increased incidence of carcinoma of the breast associated with abdominal adiposity in postmenopausal women. Am J Epidemiol 1990;131:794-803.
- Huang Z, Willett WC, Colditz GA, Hunter DJ, Manson JE, Rosner B, et al. Waist circumference, waist: Hip ratio, and risk of breast cancer in the nurses' health study. Am J Epidemiol 1999;150:1316-24.
- den Tonkelaar I, Seidell JC, Collette HJ. Body fat distribution in relation to breast cancer in women participating in the DOM-project. Breast Cancer Res Treat 1995;34:55-61.
- ;Sonnenschein E, Toniolo P, Terry MB, Bruning PF, Kato I, Koenig KL, et al. Body fat distribution and obesity in pre- and postmenopausal breast cancer. Int J Epidemiol 1999;28:1026-31.
- Bani IA, Williams CM, Boulter PS, Dickerson JW. Plasma lipids and prolactin in patients with breast cancer. Br J Cancer 1986;54:439-46.
- Schreier LE, Berg GA, Basilio FM, Lopez GI, Etkin AE, Wikinski RL, et al. Lipoprotein alterations, abdominal fat distribution and breast cancer. Biochem Mol Biol Int 1999;47:681-90.
- Høyer AP, Engholm G. Serum lipids and breast cancer risk: A cohort study of 5,207 Danish women. Cancer Causes Control 1992;3:403-8.
- ;Gaard M, Tretli S, Urdal P. Risk of breast cancer in relation to blood lipids: A prospective study of 31,209 Norwegian women. Cancer Causes Control 1994;5:501-9.
- Törnberg SA, Holm LE, Carstensen JM. Breast cancer risk in relation to serum cholesterol, serum beta-lipoprotein, height, weight, and blood pressure. Acta Oncol 1988;27:31-7.
- Manjer J, Kaaks R, Riboli E, Berglund G. Risk of breast cancer in relation to anthropometry, blood pressure, blood lipids and glucose metabolism: A prospective study within the malmö preventive project. Eur J Cancer Prev 2001;10:33-42.
- Muti P, Quattrin T, Grant BJ, Krogh V, Micheli A, Schünemann HJ, et al. Fasting glucose is a risk factor for breast cancer: A prospective study. Cancer Epidemiol Biomarkers Prev 2002;11:1361-8.
- Mink PJ, Shahar E, Rosamond WD, Alberg AJ, Folsom AR. Serum insulin and glucose levels and breast cancer incidence: The atherosclerosis risk incommunities study. Am J Epidemiol 2002;156:349-52.
- Lawlor DA, Smith GD, Ebrahim S. Hyperinsulinaemia and increased risk of breast cancer: Findings from the British women's heart and health study. Cancer Causes Control 2004;15:267-75.
- Benson EA, Holdaway IM. Regulation of insulin binding to human mammary carcinoma. Cancer Res 1982;42:1137-41.
- ;Milazzo G, Giorgino F, Damante G, Sung C, Stampfer MR, Vigneri R, et al. Insulin receptor expression and function in human breast cancer cell lines. Cancer Res 1992;52:3924-30.
- Cullen KJ, Yee D, Sly WS, Perdue J, Hampton B, Lippman ME, et al. Insulin-like growth factor receptor expression and function in human breast cancer. Cancer Res 1990;50:48-53.
- Reddy KB, Mangold GL, Tandon AK, Yoneda T, Mundy GR, Zilberstein A, et al. Inhibition of breast cancer cell growth in vitro by a tyrosine kinase inhibitor. Cancer Res 1992;52:3636-41.
- Kaaks R. Nutrition, hormones, and breast cancer: Is insulin the missing link? Cancer Causes Control 1996;7:605-25.
- Goodwin PJ, Ennis M, Pritchard KI, Trudeau ME, Koo J, Madarnas Y, et al. Fasting insulin and outcome in early-stage breast cancer: Results of a prospective cohort study. J Clin Oncol 2002;20:42-51.
- Poretsky L, Cataldo NA, Rosenwaks Z, Giudice LC. The insulin-related ovarian regulatory system in health and disease. Endocr Rev 1999;20:535-82.
- Berrino F, Muti P, Micheli A, Bolelli G, Krogh V, Sciajno R, et al. Serum sex hormone levels after menopause and subsequent breast cancer. J Natl Cancer Inst 1996;88:291-6.
- Nelson LR, Bulun SE. Estrogen production and action. J Am Acad Dermatol 2001;45:S116-24.
- McTernan PG, Anwar A, Eggo MC, Barnett AH, Stewart PM, Kumar S, et al. Gender differences in the regulation of P450 aromatase expression and activity in human adipose tissue. Int J Obes Relat Metab Disord 2000;24:875-81.
- Cauley JA, Gutai JP, Kuller LH, LeDonne D, Powell JG. The epidemiology of serum sex hormones in postmenopausal women. Am J Epidemiol 1989;129:1120-31.
- Key TJ, Appleby PN, Reeves GK, Roddam A, Dorgan JF, Longcope C, et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst 2003;95:1218-26.
- Sherif K, Kushner H, Falkner BE. Sex hormone-binding globulin and insulin resistance in African-American women. Metabolism 1998;47:70-4.
- Golden SH, Ding J, Szklo M, Schmidt MI, Duncan BB, Dobs A, et al. Glucose and insulincomponents of the metabolic syndrome are associated with hyperandrogenism in postmenopausal women: The atherosclerosis risk incommunities study. Am J Epidemiol 2004;160:540-8.
- Brismar K, Hilding A, Lindgren B. Regulation of IGFBP-1 in humans. Prog Growth Factor Res 1995;6:449-56.
- Norat T, Dossus L, Rinaldi S, Overvad K, Grønbaek H, Tjønneland A, et al. Diet, serum insulin-like growth factor-I and IGF-binding protein-3 in European women. Eur J Clin Nutr 2007;61:91-8.
- Hanaire-Broutin H, Sallerin-Caute B, Poncet MF, Tauber M, Bastide R, Rosenfeld R, et al. Insulin therapy and GH-IGF-I axis disorders in diabetes: Impact of glycaemic control and hepatic insulinization. Diabetes Metab 1996;22:245-50.
- Tollet P, Enberg B, Mode A. Growth hormone (GH) regulation of cytochrome P-450IIC12, insulin-like growth factor-I (IGF-I), and GH receptor messenger RNA expression in primary rat hepatocytes: A hormonal interplay with insulin, IGF-I, and thyroid hormone. Mol Endocrinol 1990;4:1934-42.
- Panno ML, Salerno M, Pezzi V, Sisci D, Maggiolini M, Mauro L, et al. Effect of oestradiol and insulin on the proliferative pattern and on oestrogen and progesterone receptor contents in MCF-7 cells. J Cancer Res Clin Oncol 1996;122:745-9.
- Campagnoli C, Pasanisi P, Peris C, Berrino F. Insulin-like growth factor-I and breast cancer: Epidemiological and clinical data. Breast Cancer: Prognosis, Treatment, and Prevention. In: Jorge R. Pasqualini, editors. CRC Press 2008. p. 323-42.
- Hankinson SE, Willett WC, Colditz GA, Hunter DJ, Michaud DS, Deroo B, et al. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet 1998;351:1393-6.
- Toniolo P, Bruning PF, Akhmedkhanov A, Bonfrer JM, Koenig KL, Lukanova A, et al. Serum insulin-like growth factor-I and breast cancer. Int J Cancer 2000;88:828-32.
- Baglietto L, English DR, Hopper JL, Morris HA, Tilley WD, Giles GG, et al. Circulating insulin-like growth factor-I and binding protein-3 and the risk of breast cancer. Cancer Epidemiol Biomarkers Prev 2007;16:763-8.
- Rinaldi S, Peeters PH, Berrino F, Dossus L, Biessy C, Olsen A, et al. IGF-I, IGFBP-3 and breast cancer risk in women: The European prospective investigation into cancer and nutrition (EPIC). Endocr Relat Cancer 2006;13:593-605.
- Rose DP, Haffner SM, Baillargeon J. Adiposity, the metabolic syndrome, and breast cancer in African-American and white American women. Endocr Rev 2007;28:763-77.
- Siemińska L, Wojciechowska C, Foltyn W, Kajdaniuk D, Kos-Kudła B, Marek B, et al. The relation of serum adiponectin and leptin levels to metabolic syndrome in women before and after the menopause. Endokrynol Pol 2006;57:15-22.
- Purohit A, Newman SP, Reed MJ. The role of cytokines in regulating estrogen synthesis: Implications for the etiology of breast cancer. Breast Cancer Res 2002;4:65-9.
- Vona-Davis L, Rose DP. Adipokines as endocrine, paracrine, and autocrine factors in breast cancer risk and progression. Endocr Relat Cancer 2007;14:189-206.
- Ryo M, Nakamura T, Kihara S, Kumada M, Shibazaki S, Takahashi M, et al. Adiponectin as a biomarker of the metabolic syndrome. Circ J 2004;68:975-81.
- Franceschi S, la Vecchia C, Negri E, Parazzini F, Boyle P. Breast cancer risk and history of selected medical conditions linked with female hormones. Eur J Cancer 1990;26:781-5.
- de Waard F, Baanders-van Halewijn EA. A prospective study in general practice on breast-cancer risk in postmenopausal women. Int J Cancer 1974;14:153-60.
- Moseson M, Koenig KL, Shore RE, Pasternack BS. The influence of medical conditions associated with hormones on the risk of breast cancer. Int J Epidemiol 1993;22:1000-9.
- Furberg AS, Jasienska G, Bjurstam N, Torjesen PA, Emaus A, Lipson SF, et al. Metabolic and hormonal profiles: HDL cholesterol as a plausible biomarker of breast cancer risk. The Norwegian EBBA study. Cancer Epidemiol Biomarkers Prev 2005;14:33-40.
- Schoen RE, Tangen CM, Kuller LH, Burke GL, Cushman M, Tracy RP, et al. Increased blood glucose and insulin, body size, and incident colorectal cancer. J Natl Cancer Inst 1999;91:1147-54.
- Rothman DL, Magnusson I, Katz LD, Shulman RG, Shulman GI. Quantitation of hepatic glycogenolysis and gluconeogenesis in fasting humans with 13C NMR. Science 1991;254:573-6.
- Rizza RA, Mandarino LJ, Gerich JE. Dose-response characteristics for effects of insulin on production and utilization of glucose in man. Am J Physiol 1981;240:E630-9.
- Deibert DC, DeFronzo RA. Epinephrine-induced insulin resistance in man. J Clin Invest 1980;65:717-21.
- MacGorman LR, Rizza RA, Gerich JE. Physiological concentrations of growth hormone exert insulin-like and insulin antagonistic effects on both hepatic and extrahepatic tissues in man. J Clin Endocrinol Metab 1981;53:556-9.
- Sun Y. Free radicals, antioxidant enzymes, and carcinogenesis. Free Radic Biol Med 1990;8:583-99.
- Sipe HJ Jr., Jordan SJ, Hanna PM, Mason RP. The metabolism of 17 beta-estradiol by lactoperoxidase: A possible source of oxidative stress in breast cancer. Carcinogenesis 1994;15:2637-43.
- Dandona P, Thusu K, Cook S, Snyder B, Makowski J, Armstrong D, et al. Oxidative damage to DNA in diabetes mellitus. Lancet 1996;347:444-5.
- Kitahara M, Eyre HJ, Lynch RE, Rallison ML, Hill HR. Metabolic activity of diabetic monocytes. Diabetes 1980;29:251-6.
- Armstrong D, al-Awadi F. Lipid peroxidation and retinopathy in streptozotocin-induced diabetes. Free Radic Biol Med 1991;11:433-6.


 PDF
PDF  Views
Views  Share
Share

