Trends in management of acute lymphoblastic leukemia: Influence of insurance based healthcare and treatment compliance on the outcome of adolescents and adults with acute lymphoblastic leukemia
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2016; 37(01): 32-37
DOI: DOI: 10.4103/0971-5851.177013
Abstract
Aim: In this study, we attempted to analyze the impact of insurance based health care system and treatment compliance on the outcome of adolescent and adults with acute lymphoblastic leukemia (ALL). Materials and Methods: Patients who underwent treatment for ALL during the period 2003-2011 were enrolled into this retrospective study. Patients on supportive or palliative care only and patients with age < 10 years were excluded. The hospital records and tumor registry records were studied. Patients were stratified into two groups, Group A (prior to the introduction of state health insurance [SHI], 2003-2007) and Group B (after the introduction of SHI, 2008-2011). Overall survival (OS) was calculated using Kaplan-Meier method. Results: A total of 420 patients with suspected or confirmed ALL visited our center during the study period and 179 patients (87 in Group A and 92 in Group B) were considered for inclusion. The median age in years (range) was 18 (10-57) and 18 (10-58) respectively in Groups A and B with males more than females. Median OS (95% CI) was 9 (6.7-11.2) and 12 (7.3-16.7) months in the Groups A and B respectively (P = 0.265). Poor treatment compliance in both groups was high (36% in Group A and 41% in Group B, [P = 0.107]) with lower default rates in Group B (P = 0.019). Patients with good compliance in the total study population and the individual study groups had significantly better OS. Conclusions: Insurance based health care has improved outcomes in the present study but not compliance to treatment. Significantly better OS was observed in patients with good compliance.
Publication History
Article published online:
12 July 2021
© 2016. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Aim:
In this study, we attempted to analyze the impact of insurance based health care system and treatment compliance on the outcome of adolescent and adults with acute lymphoblastic leukemia (ALL).
Materials and Methods:
Patients who underwent treatment for ALL during the period 2003-2011 were enrolled into this retrospective study. Patients on supportive or palliative care only and patients with age < 10 years were excluded. The hospital records and tumor registry records were studied. Patients were stratified into two groups, Group A (prior to the introduction of state health insurance [SHI], 2003-2007) and Group B (after the introduction of SHI, 2008-2011). Overall survival (OS) was calculated using Kaplan–Meier method.
Results:
A total of 420 patients with suspected or confirmed ALL visited our center during the study period and 179 patients (87 in Group A and 92 in Group B) were considered for inclusion. The median age in years (range) was 18 (10-57) and 18 (10-58) respectively in Groups A and B with males more than females. Median OS (95% CI) was 9 (6.7-11.2) and 12 (7.3-16.7) months in the Groups A and B respectively (P = 0.265). Poor treatment compliance in both groups was high (36% in Group A and 41% in Group B, [P = 0.107]) with lower default rates in Group B (P = 0.019). Patients with good compliance in the total study population and the individual study groups had significantly better OS.
Conclusions:
Insurance based health care has improved outcomes in the present study but not compliance to treatment. Significantly better OS was observed in patients with good compliance.
INTRODUCTION
Acute lymphoblastic leukemia (ALL) is a curable malignancy where the treatment needs to be administered for a longer duration, i.e., 2 years or more. It predominantly affects the pediatric population, although, adolescents, and young adults are also at a higher risk. The chance of survival in ALL decreases with increasing age.[1] The incidence of ALL in the pediatric population (age < 15 years) is 16.9-61.3 and 10.3-45.8 per million across various regions based cancer registries in boys and girls, respectively, as per the National Cancer Registry Programme.[2] A significant proportion of patients with ALL attending the tertiary care centers is more than 10 years of age. However, the incidence of ALL in this age group in India is not known.
Poor adherence to treatment protocol is one of the major reasons for poor outcomes in the Indian population, compared to the western population.[3,4] The reasons include lack of treatment compliance, belief in alternate medicine, lack of knowledge, cultural and social factors, high infection rates, and financial difficulties. Treatment-related mortality and morbidity due to sepsis has been shown to be a significant factor for poorer outcomes in Indian population. The high rate of infection, lack of adequate facilities for hospitalization and financial difficulties to take proper treatment have been considered as reasons for high sepsis-related mortality.[5,6]
State health insurance scheme (SHI) introduced in the state of Andhra Pradesh in the year 2007 providing much needed financial assistance to patients of low and lower middle socioeconomic group. It was expected that the outcomes and treatment compliance would improve after the introduction of SHI. In the present study conducted in a tertiary care, government center predominantly serving low and lower middle socioeconomic population, we attempted to analyze the impact of insurance based health care system and treatment compliance on outcome in adolescent and adults with ALL.
MATERIALS AND METHODS
Study design
All the patients who were diagnosed with ALL and received treatment during the period 2003-2011 were enrolled into this retrospective observational study. Patients who received supportive or palliative care only and patients with age < 10 years were excluded from the study. The hospital records and tumor registry records were studied for the details of the diagnosis, treatment given, compliance, and outcomes.
The day of entry into either out- or in-patient service has been taken on the day of entry into the study. Details of the disease diagnosis and investigations at the time of diagnosis, including complete blood picture, hepatic and renal function tests, bone marrow (BM) aspiration and biopsy, immunophenotyping, karyotyping, and cytogenetics were recorded. Details of physical examination were recorded for performance status (PS), lymphadenopathy, hepatosplenomegaly, testicular, and central nervous system involvement.
The diagnosis of ALL was based on morphological assessment of Wright–Giemsa stained BM aspirate smears confirmed by cytochemistry.[7] Immunophenotyping and karyotyping were done where ever possible for further confirmation and characterization. The diagnosis of precursor T-ALL/lymphoma was made based upon the results of a BM aspirate and/or biopsy and biopsy of other involved tissues or immunophenotyping if available.[7] Patients were considered to have T-ALL if, there are >25% BM blasts, with or without a mass lesion. For patients with a mass lesion and < 25% BM involvement, the term precursor T-cell lymphoblastic lymphoma was used. At the end of the induction phase, patients were considered to be in complete remission (CR) if the peripheral blood count was normal with no blasts in the peripheral smear or cerebrospinal fluid and ≤5% lymphoblasts in the marrow. Partial response and persistent disease were considered as 6-15% lymphoblasts and >15% lymphoblasts in the postinduction BM aspiration or biopsy smears, respectively.
Patients were stratified into two groups; Group A contains who received treatment prior to the introduction of SHI, from the year 2003 to 2007 and patients who received treatment after the introduction of SHI, i.e., 2008-2011 were considered as Group B. Only those who completed the full course of treatment for ALL were considered for analysis. Patients were treated with a protocol of physician's choice.
PS categorized as per Eastern Cooperative Oncology Group scale.[8] High total leukocyte count (TLC) was considered as TLC >50,000 cells/mm3. Anemia was defined as hemoglobin < 9 g/dl. Defaulters were defined as those patients who discontinued treatment or lost to follow-up during the treatment phase. Lack of compliance has been defined as a delay in a hospital visit and or failure to take the medicine at least for a consecutive period of 1 month, 2 or more weeks for ≥2 times in consolidation phase, a total of 3 or more months in the maintenance phase and includes defaulters. Death, default, relapse, and lost to follow-up were considered as events. Overall survival has been calculated from the day of entry into the study to the time of death or lost to follow-up.
Statistical analysis
Data were recorded on a predesigned proforma and managed using Microsoft Excel 2010 and Access 2010 database (Microsoft Corp, Redmond, WA, USA). Continuous variables were analyzed using Student's t-test and Mann–Whitney U-test as appropriate. The association between two categorical variables was evaluated by Chi-square test or Fisher's exact test as appropriate. Survival analysis was done using Kaplan–Meier method and overall survival between the two groups was compared using log-rank test. All tests were two-tailed; a P < 0.05 was considered as significant.
Statistical analysis was done using Statistical software PASW Statistics 18, Release 18.0.0, (IBM SPSS Statistics, Somers, NY, USA).
RESULTS
A total of 420 patients with suspected or confirmed ALL visited our center from January 2003 to December 2011. Among them, 179 patients (87 in Group A and 92 in Group B) who received treatment during the study period were considered for inclusion as shown in the study design [Figure 1].
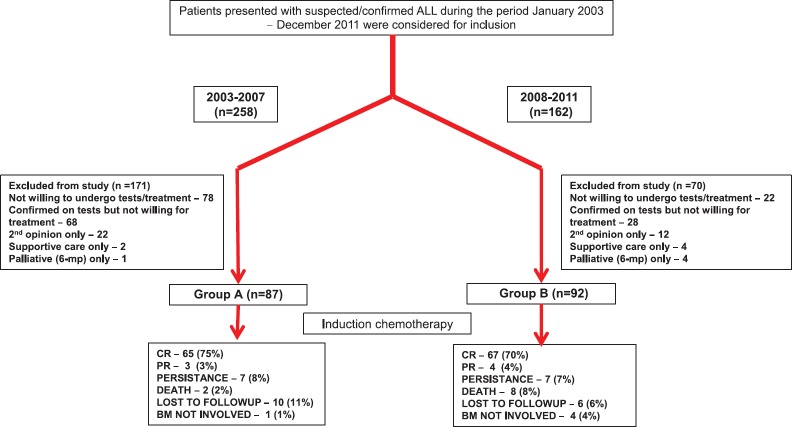
| Fig. 1 Study design. ALL – Acute lymphoblastic leukemia; CR – Complete response; PR – Partial response; BM – Bone marrow
The baseline clinical and laboratory characteristics of patients in both groups were given in Table 1. The median age of patients in both groups was 18 years, with males more than females. Group A predominantly consisted of patients who received treatment without cashless or reimbursable government or employer-provided insurance facility. The majority of patients in Group B received treatment with assistance from SHI in addition to other insurance schemes and government employees with reimbursement facility.
Table 1
Baseline demographic and disease characteristics of patients in the study
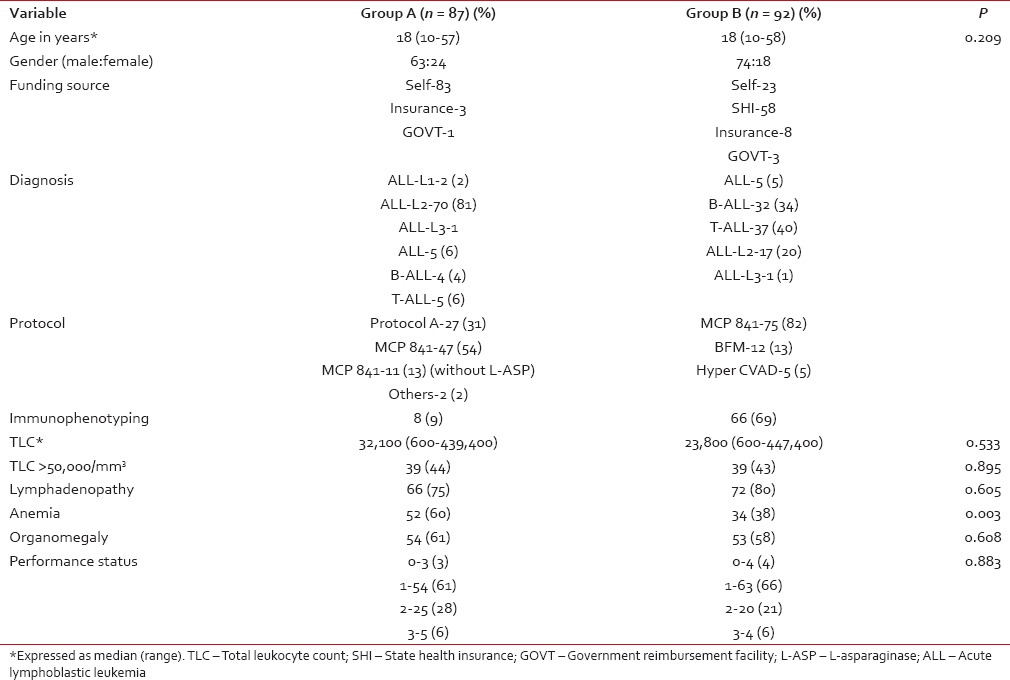
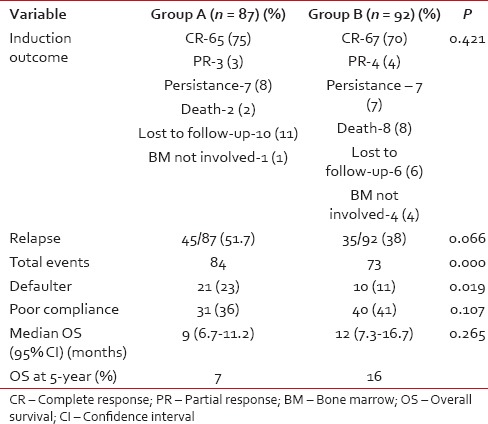
Table 2
Comparison of treatment outcomes in the study groups
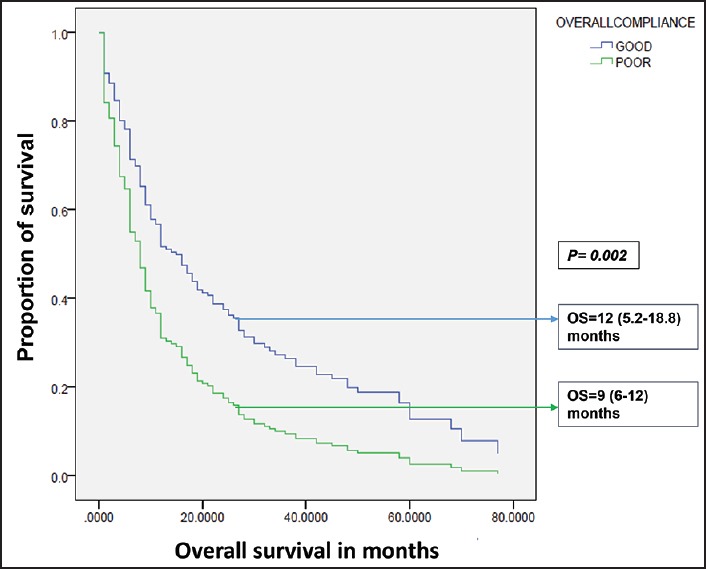
| Fig. 2 Influence of compliance on overall survival in total study group. OS – Overall survival; OS expressed as median (95% confidence interval)
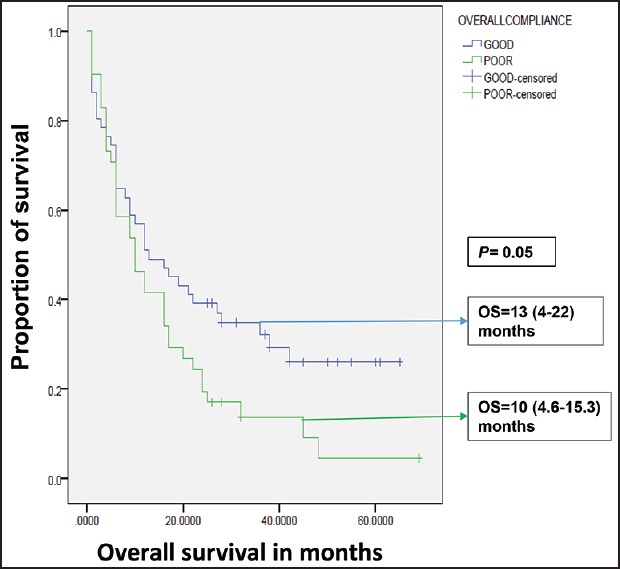
| Fig. 4 Influence of compliance on overall survival in Group B. OS – Overall survival; OS expressed as median (95% confidence interval)
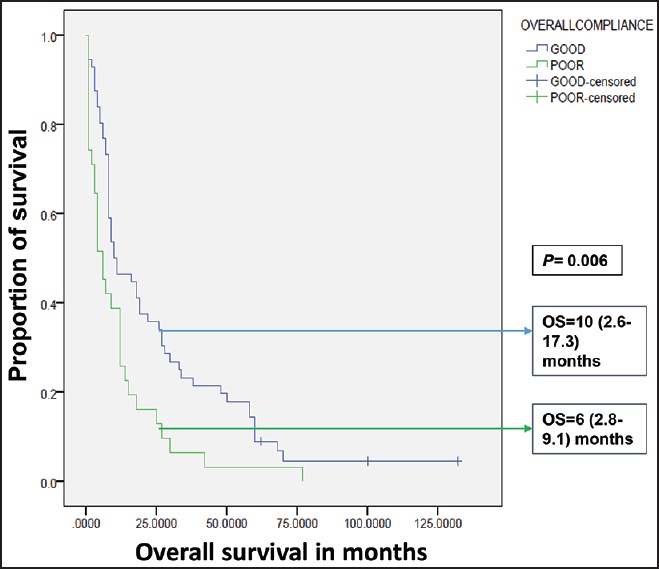
| Fig. 3 Influence of compliance on overall survival in Group A. OS – Overall survival; OS expressed as median (95% confidence interval)
DISCUSSION
In this study, the 3 year survival rates, as well as the median survival, did show improvement albeit, not statistically significant, in the Group B. The comparison of two groups over different time periods has shown that the Group B, which received greater insurance support, had better utilization of modern diagnostic and therapeutic facilities. Group B had fewer defaulters, a lesser number of relapses and significantly lesser events compared to the Group A. The overall compliance were similar with poor compliance rates in both groups. In both groups, compliance to treatment has improved OS significantly. The present study depicts the picture in which, in spite of the insurance based system, predominantly provided by the government, health care in India was still handicapped by the poor treatment compliance leading to poorer results.
In the absence of universally accepted the definition of poor treatment compliance in ALL, we have chosen the current method to define poor compliance. In a study presented at our institute at SIOP 2013,[9] misconception about childhood cancer as an incurable disease amounted to maximum default rates followed by financial constraints and switching over to alternate health systems. The poorer outcomes noticed in the present study in spite of the provision of treatment under insurance based healthcare could be due to poor risk factors present in both the study groups at the baseline such as high TLC, high tumor burden at the baseline (lymphadenopathy, hepatosplenomegaly) as noticed in various studies.[4,10] Utilization of methods to diagnose high-risk subset within the study population such as day 7 prednisone response, minimal residual disease analysis were not used in the present study due to the lack of such provisions in the protocols followed. Treatment intensification strategies including allogeneic stem cell transplantation (SCT) in the high-risk population would have improved outcomes.[11,12,13] Less than a third of relapsed patients received second-line treatment leading to decreased overall survival in the present study.
The study population included in the present study consisted of older children, adolescents and adults who were described as high-risk population by National Cancer Institute criteria: (1) This age group of patients with ALL often underrepresented in the studies from India contributes to a significant proportion of patients with ALL. To our knowledge, the present study is the third study from India reporting on the outcomes of adult ALL.
Comparing OS of the patients in Group B with that of developed western world shows inferior survival rates. The 5 years OS in various studies and SEER database ranges from 30% to 40%.[11,14,15] However, significant differences in the OS of various ethnicities were evident in SEER data during the period 1997-2002, African Americans had 5 years OS of 18% only compared to 33% of the white population and the cumulative OS for all ethnicities was 31.6%.[15] The recent data during the period 2003-2008 showed improvement in the OS in these groups and was largely due to improved treatment stratification and allogeneic SCT. Compared to the western population, a higher proportion of present study subjects had adverse risk factors as noticed by various studies.[10,11,14] The 5 years OS in the present study population is 18% in the Group B. This indicates the poor outcomes in the Indian population and requirement of strategies to improve treatment outcomes.
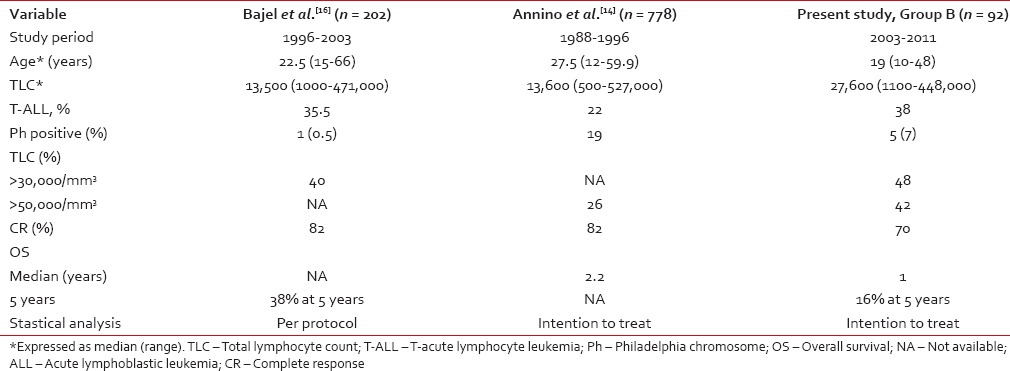
Table 3 shows a comparison of Group B in this study with other published studies from Vellore and GIMEMA group.[14,16] The OS and CR at the end of first induction in the Group B were inferior to the results of the Vellore study and GIMEMA study [Table 3]. However, protocol followed in the Vellore study was modified German ALL (GMALL) protocol and predominant protocol in the present study was MCP 841. Only the patients who have achieved CR were considered for further analysis in the Vellore study.[16] Being a government hospital, the present study had a patient population from lower and lower middle socioeconomic status and had high default rates and poor treatment compliance. The compliance rate for treatment in Vellore study was not available. Another study from Mumbai, consisting of a small sample size of 42 patients was reported from India.[17] The protocol followed was MCP 841, and it reported 5 years OS of 42%. The necessity to take prolonged treatment in disease such as ALL needs to look past the treatment care provided at the hospital level and need community level support provided by health care workers at primary health center level.
Table 3
Comparison with the other published studies
 The present study is a retrospective study and uniform treatment protocol was not followed in both groups. The comparison of patients who took treatment under insurance coverage with the patients who paid for their treatment during the same period would have been optimal. However, the SHI covered two-third population who visited our center during the period 2008-2012 and most of the patients in the other one-third had other insurance or reimbursement facilities. The improvement noticed in the 3 years OS as well as the median OS was not statistically significant. This observation could be due to the lower sample size in the study and a study with larger sample size could throw further light on this observation.
The present study is a retrospective study and uniform treatment protocol was not followed in both groups. The comparison of patients who took treatment under insurance coverage with the patients who paid for their treatment during the same period would have been optimal. However, the SHI covered two-third population who visited our center during the period 2008-2012 and most of the patients in the other one-third had other insurance or reimbursement facilities. The improvement noticed in the 3 years OS as well as the median OS was not statistically significant. This observation could be due to the lower sample size in the study and a study with larger sample size could throw further light on this observation.CONCLUSIONS
The insurance based health care system has definitely improved the treatment outcomes and utilization of modern diagnostic facilities in the present study population. Although, default rates have decreased in the group with state health insurance, the overall compliance rates were similar in both groups. Poor compliance to treatment resulted in inferior survival and persisted in the present study in spite of the provision of financial assistance and requires consideration of alternative strategies to improve compliance.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
I sincerely acknowledge departmental staff, patients, and my family members for their contribution towards the study.


 PDF
PDF  Views
Views  Share
Share

