A Case of Multifocal Eosinophilic Granuloma Involving Spine and Pelvis in a Young Adult: A Radiopathological Correlation
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(04): 555-558
DOI: DOI: 10.4103/ijmpo.ijmpo_130_16
Abstract
We present a case of multiple osteolytic lesions in a 28-year-old adult who presented with headache, back pain, and hip pain of 6 months. There was no history of localized swelling or rise of temperature, no history of weight loss or evening rise of temperature. On examination, there were no focal neurological deficits. Routine laboratory investigations, including total leukocyte counts, differential leukocyte counts, hemoglobin, and platelet counts, were within normal limits. There was a borderline elevation of erythrocyte sedimentation rate. Non enhancedcomputer tomography (NECT) demonstrated no abnormality in the brain or skull bones. However, incidentally, a lytic lesion involving the third cervical (C3) vertebral body and the neural arch was detected which also demonstrated a soft tissuecomponent adjacent to the lytic lesion. These findings warranted further work up; and magnetic resonance imaging of whole spine and pelvis was performed that revealed multiple bony lesions involving the cervical vertebrae, head and neck, bilateral femur, sacrum, and iliac bones. Computed tomography-guided biopsy was performed from the C3 vertebral lytic lesion which showed features of eosinophilic granuloma on histopathological evaluation.
Publication History
Article published online:
04 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used forcommercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
We present a case of multiple osteolytic lesions in a 28-year-old adult who presented with headache, back pain, and hip pain of 6 months. There was no history of localized swelling or rise of temperature, no history of weight loss or evening rise of temperature. On examination, there were no focal neurological deficits. Routine laboratory investigations, including total leukocyte counts, differential leukocyte counts, hemoglobin, and platelet counts, were within normal limits. There was a borderline elevation of erythrocyte sedimentation rate. Non enhanced computer tomography (NECT) demonstrated no abnormality in the brain or skull bones. However, incidentally, a lytic lesion involving the third cervical (C3) vertebral body and the neural arch was detected which also demonstrated a soft tissue component adjacent to the lytic lesion. These findings warranted further work up; and magnetic resonance imaging of whole spine and pelvis was performed that revealed multiple bony lesions involving the cervical vertebrae, head and neck, bilateral femur, sacrum, and iliac bones. Computed tomography-guided biopsy was performed from the C3 vertebral lytic lesion which showed features of eosinophilic granuloma on histopathological evaluation.
Introduction
Eosinophilic granuloma (EG) is the least severe form of three clinical variants of Langerhans cell histiocytosis.[1] It is characterized by proliferation of CD1a dendritic cells.[2] EG accounts for <1 href="https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5759083/#ref3" rid="ref3" class=" bibr popnode tag_hotlink tag_tooltip" id="__tag_659254168" role="button" aria-expanded="false" aria-haspopup="true" xss=removed>3] We present a case of multiple osteolytic lesions in a 28-year-old adult who presented with headache, back pain, and hip pain of 6 months.
Case Report
We present a case of a 28-year-old male who presented with headache, back pain, and hip pain of 6 months. There was no history of localized swelling or rise of temperature and no history of weight loss or evening rise of temperature. On examination, there were no focal neurological deficits. Routine laboratory investigations, including total leukocyte counts, differential leukocyte counts, hemoglobin, and platelet counts, were within normal limits. There was a borderline elevation in erythrocyte sedimentation rate. The computed tomography (CT) scan of the cervical spine revealed an ill-defined lytic lesion in the left half of the C3 vertebral body with soft tissue infiltration [Figure 1]. Magnetic resonance imaging (MRI) demonstrated these lesions as iso-hyperintense on T2/fat sat and hypointense on T1-weighted with soft tissue component encasing left exiting nerve root and mildly enhancing heterogeneous adjacent soft tissue component and restricted diffusion [Figures [Figures11 and and2].2]. The MRI and plain radiographs of the calvarium and the spine and pelvis demonstrated multiple lytic lesions in the pelvic bone and absence of calvarial lesions [Figure 2]. However, the skeletal survey did not reveal any other lesions. CT-guided biopsy was performed from C3 vertebral body as it showed features of active lesion. Histopathology showed fibro-collagenous tissue with thin-walled blood vessels infiltrated by eosinophilic micro-abscesses along with sheets of eosinophils that were features are of EG [Figure 3].
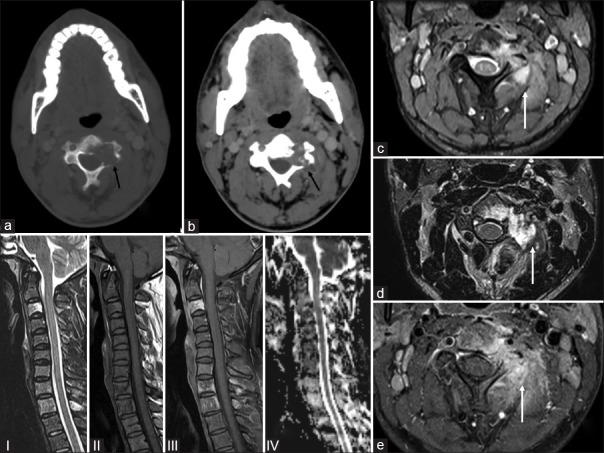
| Figure 1:A 28-year-old male presenting with pain (a) axial computed tomography images (bone window) showing an ill-defined lytic lesion in the left half of the C3 vertebral body (black arrow) and (b soft tissue window) soft tissue infiltration (black arrow) confirmed on the (c) gradient T2 axial magnetic resonance imaging, (d) axial T2 magnetic resonance imaging, (e) fat suppressed images axial magnetic resonance images (white arrows) with soft tissue component encasing left exiting nerve root and shows mildly enhancing heterogeneous adjacent soft tissue component and restricted diffusion. (and seen on the sagittal magnetic resonanceimages (I-T2, II-T1, III-fat suppressed, IV-diffusion images) where he C3 body is iso-hyperintense on T2/fat sat and hypointense on T1 weighted
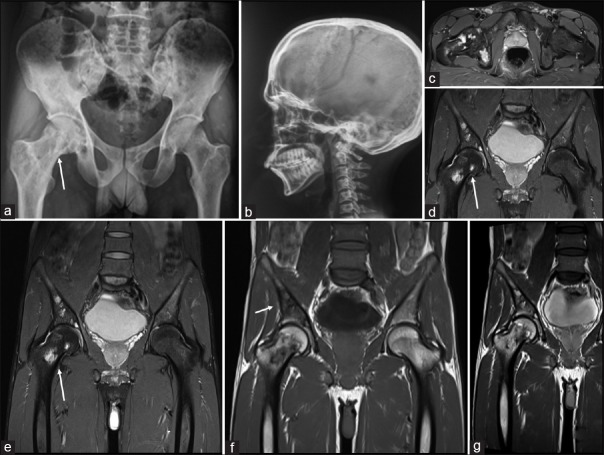
| Figure 2:A 28-year-old male presenting with pain: (a) plain radiographs of the pelvis (white arrow) and (b) plain Radiograph Skull lateral shows no calvarial lesions but (c) magnetic resonance imaging axial fat suppressed ill-defined lytic lesions in the trochanter (d and e) coronal fat suppressed magnetic resonance images shows multiple hyperintense areas in the femoral head, trochanter (white arrows) (f) magnetic resonance imaging coronal T1 and (g) magnetic resonance imaging coronal T2 show lesions also in bilateral sacral alae, sacral vertebrae and bilateral iliac bones
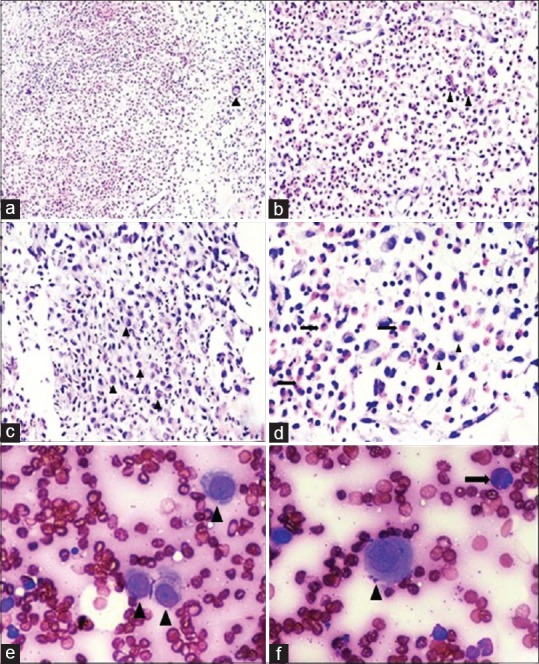
| Figure 3:Guided lesional biopsy of the lytic lesion present in the C3 vertebra in a 28-year-old man: (a) Varying proportions of Langerhans cells, multinucleated giant cells (arrow head), eosinophils, and small lymphocytes (H and E, ×40). (b) Numerous eosinophils with formation of eosinophilic microabscesses (arrow heads) (H and E, ×100). (c and d) Langerhans cells exhibiting moderate degree of cytologic atypia with dented, folded, and grooved nuclei (arrow heads). Neutrophils and eosinophils (arrows) are also seen accompanying the Langerhans cells (H and E, ×200). (e and f) Fine-needle aspiration: Langerhans cells (arrow heads) having abundant pale to intensely eosinophilic cytoplasm with oval nucleus and prominent longitudinal grooving. Few eosinophils (arrow) are seen scattered in the background (H and E, ×400)
Discussion
EG is an immune-proliferative disorder characterized by abnormal proliferation of CD1a dendritic cells.[2] The usual presentation is either as a solitary lesion that rarely requires clinical attention or as a multisystem, life-threatening disease that necessities an aggressive therapy.[3]
The occurrence of EG in vertebral body ranges between 7% and 15% of cases. It is a childhood disease with the most common age at presentation being <10 href="https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5759083/#ref4" rid="ref4" class=" bibr popnode tag_hotlink tag_tooltip" id="__tag_659254163" role="button" aria-expanded="false" aria-haspopup="true" xss=removed>4]
Skeletal involvement may be widespread, but more common sites are the skull, spine, pelvis, ribs, mandible, femur, tibia, and humerus with rare involvement of bones of the hands and feet.[2] Pathological fractures may ensue due to the lytic lesions.[4] The disease may show spontaneous regression.[4]
The spine lesions are generally asymptomatic but the clinical symptoms may be in the form of local pain, fever or weight loss.[5] In children, thoracic (54%), lumbar (35%), and cervical (11%) spines are affected accordingly. In adults, cervical spine (47%) involvement is much more common than the thoracic (33%), and the lumbar (20%) spines.[4] In a study by Islinger et al.[6] that included 541 patients older than 21 years, the thoracic spine was almost exclusively involved.[6]
Radiological images of the skull may reveal punched out lytic lesions and shows bevelled appearance since there is involvement of both inner as well as outer tables. Uninvolved bone within the punched out lesions appear in the form of button sequestrum.[5] Vertebra plana are known to occur in the vertebral body secondary to collapse. Adjacent sclerosis may be noted in the healing stage.[7]
Radiological differential diagnosis, EG should be differentiated from variety of bone conditions such as aneurysmal bone disease, osteomyelitis, Ewing's sarcoma, osteoblastoma, Gaucher's disease, acute leukemia, lymphoma, plasmacytoma, chordoma, and metastatic tumor.[2,4] Most of these conditions radiologically presents as vertebra plana. Difficulty of diagnosing EG by radiograph increases in areas with more complex anatomy such as posterior elements of vertebral bodies. These cases further requires computer tomography scan to eliminate false-negative cases.[2]
CT scan of EG of the vertebral body may have bubbly, lytic, expansile lesions of both the body, and posterior elements commonly involving thoracic and lumbar spines. However, the intervertebral disc height is usually maintained.[7]
MRI is useful in delineating the amount of marrow and soft tissue involvement and to assess the amount of peri-tumoral edema.[7,8] The lesions appear as T1 hypointense, T2 and short tau inversion recovery hyperintense due to soft tissue component and marrow edema.[8] An endosteal rim of low signal intensity may be seen due to sclerosis which is indicative of the healing stage. Lesion with high cellularity show high signal on T2 and contrast-enhanced T1 images thereby suggesting active disease. Biopsy should be performed from these lesions as it yields good specimen for histopathological examination. The healed lesions will have fibrosis and will show low signal intensity on T2 and post contrast T1 images.[8]
The approach to the diagnosis of EG is always multicentric that requires correlation of hematologic, radiological and histopathological findings. The case, on cytological smear, can be termed EG accurately only when the cytopathologist is aware of this entity and also it warrants a high index of suspicion since clinical diagnosis of EG is not possible in most of the cases. The smears are highly cellular comprising of sheets and singly scattered Langerhan's cells and are admixed with eosinophils, few neutrophils, plasma cells, histiocytes, and multinucleated giant cells. Morphological identification of Langerhan's cells will clinch the diagnosis with typical dendritic-like cytoplasmic processes, nuclear grooves and pseudo-inclusions. They also exhibit frequent mitotic figures.[9,10] However, these cells most often lack the cytoplasmic processes and can show only fewer nuclear grooves.[10] The background eosinophils may draw the attention of the pathologist to search for Langerhan's cells in cases of EG. Many studies have shown that the eosinophil number need not be strikingly present but can be sparse.[9]
Cytological differential diagnosis and histopathological diagnosis of EG requires ruling out much more common conditions such as osteomyelitis, Ewing's sarcoma, non-Hodgkin lymphoma (NHL), sinus histiocytosis with massive lymphadenopathy (SHML), Hodgkin lymphoma, leukemia, and metastatic tumors with common primary being lung tumors.[9,11] In acute conditions such as osteomyelitis, neutrophils are seen in abundance and reactive histiocytes are seen commonly without any atypical cytological features. In chronic osteomyelitis, the predominant cell types are plasma cells and lymphocytes that are not commonly encountered in EG.[9] The histiocytes in SHML have abundant cytoplasm with prominent nucleoli and exhibit hemophagocytosis.[12] Malignancies such as Hodgkin lymphoma, NHL, leukemia, or metastasis can show proliferation of Langerhan's cells. Malignant melanoma and papillary carcinoma of thyroid can show the presence of histiocytes with nuclear grooves or pseudo-inclusions. However, none of these histiocytes show any atypical features.[9]
Histopathological examination of EG is essential in suspected cases of spinal EG where the typical features on radiological evaluation are not seen. Those cases with atypical features such as presence of soft tissue extension, involvement of intervertebral disc, or presence of neurological involvement. Tissue biopsy is confirmatory with the presence of different cell populations such as eosinophils, neutrophils, histiocytes, lymphocytes, plasma cells, and multinucleated giant cells. Patients with vertebra plana calls in many differential diagnoses, including Ewing sarcoma, osteosarcoma, leukemia, and lymphoma. A systematic diagnostic approach including biopsy evaluation is required with a stringent follow up. In cases of multicentric disease, the most accessible location should be selected for the biopsy.[13]
Conclusion
EG can be diagnosed by clinical examination and characteristic radiological findings that can be supplemented by fine-needle aspiration cytology. However, EG on cytology can only be diagnosed when one is aware about its characteristic cytological features findings and pose a diagnostic challenge.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Lichtenstein L, Jeffe HL. Eosinophilic granuloma of bone: With report of a case. Am J Pathol 1940;16:595-604.
- Garg B, Sharma V, Eachempati KK, Malhotra R, Bhan S. An unusual presentation of eosinophilic granuloma in an adult: A case report. J Orthop Surg 2006;14:81-3.
- Buchmann L, Emami A, Wei JL. Primary head and neck Langerhans cell histiocytosis in children. Otolaryngol Head Neck Surg 2006;135:312-7.
- Reddy PK, Vannemreddy PS, Nanda A. Eosinophilic granuloma of spine in adults: A case report and review of literature. Spinal Cord 2000;38:766-8.
- David R, Oria RA, Kumar R, Singleton EB, Lindell MM, Shirkhoda A, et al. Radiologic features of eosinophilic granuloma of bone. AJR Am J Roentgenol 1989;153:1021-6.
- Islinger RB, Kuklo TR, Owens BD, Horan PJ, Choma TJ, Murphey MD, et al. Langerhans' cell histiocytosis in patients older than 21 years. Clin Orthop Relat Res 2000;379:231-5.
- Azouz EM, Saigal G, Rodriguez MM, Podda A. Langerhans' cell histiocytosis: Pathology, imaging and treatment of skeletal involvement. Pediatr Radiol 2005;35:103-15.
- Moon TY, Lee J, Lee IS, Choi KU, Chae JM, Kim J, et al. MRI and histopathologic classification of Langerhans cell histiocytosis. Curr Med Imaging Rev 2009;5:14-8.
- Kumar N, Sayed S, Vinayak S. Diagnosis of Langerhans cell histiocytosis on fine needle aspiration cytology: A case report and review of the cytology literature. Patholog Res Int 2011;2011:439518.
- Jayaram G. Cytoplasmic processes as a diagnostic aid in Langerhans cell histiocytosis. Acta Cytol 2007;51:833-4.
- Ueda Y, Murakami H, Demura S, Kawahara N, Tomita K, Tsuchiya H. Eosinophilic granuloma of the lumbar spine in an adult. Orthopedics 2012;35:818-21.
- Kakkar S, Kapila K, Verma K. Langerhans cell histiocytosis in lymph nodes. Cytomorphologic diagnosis and pitfalls. Acta Cytol 2001;45:327-32.
- Garg S, Mehta S, Dormans JP. Langerhans cell histiocytosis of the spine in children long-term follow-up. J Bone Joint Surg 2004;86:1740-50.

| Figure 1:A 28-year-old male presenting with pain (a) axial computed tomography images (bone window) showing an ill-defined lytic lesion in the left half of the C3 vertebral body (black arrow) and (b soft tissue window) soft tissue infiltration (black arrow) confirmed on the (c) gradient T2 axial magnetic resonance imaging, (d) axial T2 magnetic resonance imaging, (e) fat suppressed images axial magnetic resonance images (white arrows) with soft tissue component encasing left exiting nerve root and shows mildly enhancing heterogeneous adjacent soft tissue component and restricted diffusion. (and seen on the sagittal magnetic resonanceimages (I-T2, II-T1, III-fat suppressed, IV-diffusion images) where he C3 body is iso-hyperintense on T2/fat sat and hypointense on T1 weighted

| Figure 2:A 28-year-old male presenting with pain: (a) plain radiographs of the pelvis (white arrow) and (b) plain Radiograph Skull lateral shows no calvarial lesions but (c) magnetic resonance imaging axial fat suppressed ill-defined lytic lesions in the trochanter (d and e) coronal fat suppressed magnetic resonance images shows multiple hyperintense areas in the femoral head, trochanter (white arrows) (f) magnetic resonance imaging coronal T1 and (g) magnetic resonance imaging coronal T2 show lesions also in bilateral sacral alae, sacral vertebrae and bilateral iliac bones

| Figure 3:Guided lesional biopsy of the lytic lesion present in the C3 vertebra in a 28-year-old man: (a) Varying proportions of Langerhans cells, multinucleated giant cells (arrow head), eosinophils, and small lymphocytes (H and E, ×40). (b) Numerous eosinophils with formation of eosinophilic microabscesses (arrow heads) (H and E, ×100). (c and d) Langerhans cells exhibiting moderate degree of cytologic atypia with dented, folded, and grooved nuclei (arrow heads). Neutrophils and eosinophils (arrows) are also seen accompanying the Langerhans cells (H and E, ×200). (e and f) Fine-needle aspiration: Langerhans cells (arrow heads) having abundant pale to intensely eosinophilic cytoplasm with oval nucleus and prominent longitudinal grooving. Few eosinophils (arrow) are seen scattered in the background (H and E, ×400)
References
- Lichtenstein L, Jeffe HL. Eosinophilic granuloma of bone: With report of a case. Am J Pathol 1940;16:595-604.
- Garg B, Sharma V, Eachempati KK, Malhotra R, Bhan S. An unusual presentation of eosinophilic granuloma in an adult: A case report. J Orthop Surg 2006;14:81-3.
- Buchmann L, Emami A, Wei JL. Primary head and neck Langerhans cell histiocytosis in children. Otolaryngol Head Neck Surg 2006;135:312-7.
- Reddy PK, Vannemreddy PS, Nanda A. Eosinophilic granuloma of spine in adults: A case report and review of literature. Spinal Cord 2000;38:766-8.
- David R, Oria RA, Kumar R, Singleton EB, Lindell MM, Shirkhoda A, et al. Radiologic features of eosinophilic granuloma of bone. AJR Am J Roentgenol 1989;153:1021-6.
- Islinger RB, Kuklo TR, Owens BD, Horan PJ, Choma TJ, Murphey MD, et al. Langerhans' cell histiocytosis in patients older than 21 years. Clin Orthop Relat Res 2000;379:231-5.
- Azouz EM, Saigal G, Rodriguez MM, Podda A. Langerhans' cell histiocytosis: Pathology, imaging and treatment of skeletal involvement. Pediatr Radiol 2005;35:103-15.
- Moon TY, Lee J, Lee IS, Choi KU, Chae JM, Kim J, et al. MRI and histopathologic classification of Langerhans cell histiocytosis. Curr Med Imaging Rev 2009;5:14-8.
- Kumar N, Sayed S, Vinayak S. Diagnosis of Langerhans cell histiocytosis on fine needle aspiration cytology: A case report and review of the cytology literature. Patholog Res Int 2011;2011:439518.
- Jayaram G. Cytoplasmic processes as a diagnostic aid in Langerhans cell histiocytosis. Acta Cytol 2007;51:833-4.
- Ueda Y, Murakami H, Demura S, Kawahara N, Tomita K, Tsuchiya H. Eosinophilic granuloma of the lumbar spine in an adult. Orthopedics 2012;35:818-21.
- Kakkar S, Kapila K, Verma K. Langerhans cell histiocytosis in lymph nodes. Cytomorphologic diagnosis and pitfalls. Acta Cytol 2001;45:327-32.
- Garg S, Mehta S, Dormans JP. Langerhans cell histiocytosis of the spine in children long-term follow-up. J Bone Joint Surg 2004;86:1740-50.


 PDF
PDF  Views
Views  Share
Share

