A Case Report of Krukenbergs Tumor with Cutaneous Seeding
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(01): 67-69
DOI: DOI: 10.4103/0971-5851.203504
Abstract
A 47-year-old female patient presented with painless skin colored and erythematous papules coalesced to form plaques over lower abdomen for 10 days. She had undergone exploratory laparotomy with hysterectomy and bilateral oophorectomy 1 month ago, and histopathology was reported as Krukenbergs tumor. She was getting evaluated for primary, when she was referred to dermatology. A clinical diagnosis of cutaneous infiltration of tumor was made, and biopsy was done from a representative lesion which showed features suggestive of metastatic poorly differentiated adenocarcinoma. In the majority of cases in the past, cutaneous metastasis is seen much later in the course of the disease. High degree of suspicion and histopathology was helpful in the diagnosis of underlying malignancy in our patient.
Publication History
Article published online:
06 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
A 47-year-old female patient presented with painless skin colored and erythematous papules coalesced to form plaques over lower abdomen for 10 days. She had undergone exploratory laparotomy with hysterectomy and bilateral oophorectomy 1 month ago, and histopathology was reported as Krukenbergs tumor. She was getting evaluated for primary, when she was referred to dermatology. A clinical diagnosis of cutaneous infiltration of tumor was made, and biopsy was done from a representative lesion which showed features suggestive of metastatic poorly differentiated adenocarcinoma. In the majority of cases in the past, cutaneous metastasis is seen much later in the course of the disease. High degree of suspicion and histopathology was helpful in the diagnosis of underlying malignancy in our patient.
Introduction
Cutaneous metastasis of malignant tumor is often a rare event and has been reported to occur in 0.7%–9% of patients with malignancy.[1] Skin is often an uncommon site of metastasis from internal malignancy. The presence of cutaneous metastasis implies a systemic spread of disease and poor prognosis with early fatal termination, with an average survival of 3–18 months.[2] Usually, there is a long time interval between the diagnosis of the primary malignancy and the recognition of the skin metastases. Here, we present a case of Krukenbergs tumor with metastasis to skin occurring earlier than the usual and subsequent detection of primary.
Case Report
A 47-year-old female patient presented to the oncology outpatient department with the previous history of pain abdomen and vomiting for which exploratory laparotomy and hysterectomy was done 1 month ago, and histopathology was reported as Krukenberg's tumor. She was referred to dermatology for raised lesions over the lower abdomen since 10 days. On examination, there were multiple skin-colored and erythematous papules coalesced to form plaques over lower abdomen, firm to hard in consistency, surrounding skin had a doughy feel [Figure 1]. A provisional diagnosis of cutaneous infiltration of tumor was made. Biopsy of representative lesion showed diffuse infiltration of tumor cells in cords and singles, with pleomorphic round to oval nucleus, high N:C ratio and vesicular chromatin in the dermis, along with signet ring-like cells and lymphoplasmacytic infiltrate around blood vessels suggestive of metastatic poorly differentiated adenocarcinoma [Figure 2]. Magnetic resonance imaging scan showed thickening of skin with multiple small enhancing nodules in subcutaneous plane around the surgical scar of anterior abdominal wall along with pulmonary metastasis. Upper gastrointestinal (GI) endoscopy showed a friable hypertrophic growth at gastroesophageal junction, which on biopsy showed features suggestive of poorly differentiated adenocarcinoma [Figure 3]. The patient was started on chemotherapy, and unfortunately our patient succumbed to metastatic disease after 2 months of presentation.
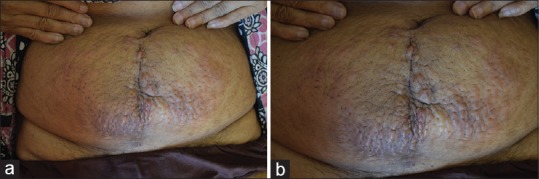
| Figure 1(a) Erythematous to hyperpigmented infiltrated nodules and plaques, showing peau dæorange appearance. (b) Close up view
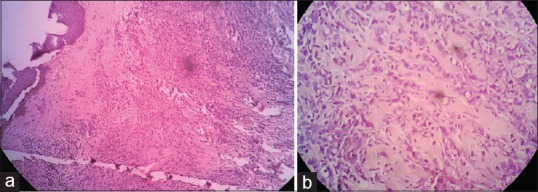
| Figure 2(a) Low power: Dermis shows diffuse infiltration of tumor cells. Lymphoplasmacytic infiltrate around blood vessels. (b) High power: Dermal tumor cells are present in cords and singles, with pleomorphic round to oval nucleus, high N:C ratio, and vesicular chromatin. Signet ring-like cells are present
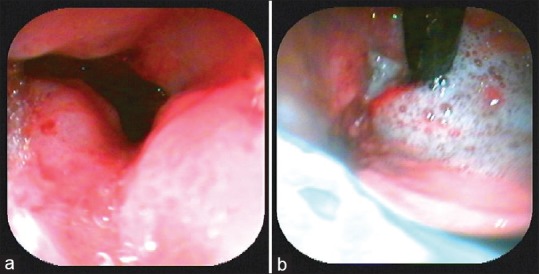
| Figure 3(a) Endoscopic view of gastroesophageal junction: Friable hypertrophic growth, which on biopsy showed features suggestive of poorly differentiated adenocarcinoma. (b) Endoscopic view of fundus of the stomach
Discussion
Cutaneous metastasis usually occur late in the course of malignancy. Occasionally, skin metastasis may be detected simultaneously or sometimes even before the diagnosis of a primary tumor. They occur mainly in the age group between 50 and 70 years.[3] The average time interval between the diagnosis of primary malignancy and skin metastasis is approximately around 33 months. The average survival is 7.5 months following diagnosis, with around 50% of patients with skin metastasis die within the first 6 months.[4] The tumor cells spread to the skin through lymphatic and/or hematogenous route. However, a direct extension to the skin or accidental implantation in surgical wounds and surgical tracts is reported as well.[2]
Cutaneous metastasis of esophageal adenocarcinoma are exceedingly rare and occur in only 1% of patients. They occur most commonly on the abdominal wall as local skin metastasis or seeding, most often after invasive procedures for diagnostic or therapeutic purposes.[5] Currently, adenocarcinoma occurs in more than 50% of all new cases of esophageal carcinoma, and its pathogenesis is linked to gastroesophageal reflux disease.[6]
The biologic basis of cutaneous metastasis is incompletely understood. It occurs as a complex interaction among genetic factors, epigenetic events, and host responses.[4] The tumor needs to detach from the primary tumor, invade a blood or lymphatic vessel, circulate in them, extravasate, reattach in a distant site, finally, survive and proliferate at the secondary site.[1] Studies have shown that tumors have a selective ability to metastasize to a certain site. They may be the first manifestation of unsuspected occult malignancy or an indicator of recurrence of malignancy after treatment or failure of ongoing therapy.[3]
Metastatic cutaneous lesions may have a variety of presentation, but most of them fall under one of three main types of lesions nodules, plaques, and inflammatory telengiectatic lesions.[7] They usually present as painless nodule within the dermis and subcutaneous tissue with an intact, uninvolved elevated epidermis. Skin metastasis in esophagogastric carcinoma may occur as cutaneous nodules, infiltrated skin plaques, cellulitis, carcinoma erysipeloides, carcinoma en cuirasse, or as large cauliflower-like papillomatous mass.[4]
According to histopathology, they are divided as adenocarcinoma, squamous cell carcinoma, undifferentiated carcinoma, and other miscellaneous types.[8] A large number of tumor cells are seen in the deep reticular dermis and subcutaneous tissue, forming glands, clusters or strands of tumor cells in an abundance of fibrous stroma. Tumor cells are seen in lymphatic and blood vessels, and also lining up between the collagen bundles. The epidermis is usually separated from the tumor cell in the dermis, with a tumor-free grenz zone. They usually react to mucicarmine, alcian blue, periodic acid Schiff, CDX2, CK7, CK20, carcinoembryoic antigen, and epithelial membrane antigen. In most cases, the histological features of cutaneous metastasis resemble the underlying primary.[4] Cutaneous lesions are easily accessible for histopathology, and skin biopsy from the suspected metastasis helps in confirmation of diagnosis. Although they do not determine the site of primary tumor may produce some clues which are helpful in diagnosis. For example, in our case, the presence of signet ring-like cells in the histopathology of skin biopsy acted as a confirmatory in evaluation for primary indicating that it is from the GI tract.
The outcome of the cutaneous metastasis varies depending on the primary malignancy but the presence of cutaneous infiltration by the tumor cells signifies widespread disease and a high mortality rate. Esophageal carcinoma has one of the highest mortality rates of all cancers.[6] Esophageal carcinoma is usually diagnosed at an advanced stage, when the cure is almost unlikely. It carries a poor prognosis with a 5-year survival rate of around 8% and a median survival of 9 months.[5]
Conclusion
It is very uncommon for cutaneous seeding to present at the time of diagnosis of internal malignancy. This case highlights the importance of high degree of suspicion and histopathology leading to diagnosis of underlying malignancy in cutaneous metastasis. If appropriate treatment is done following diagnosis of cutaneous metastasis it may relatively prolong patient survival.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Rao R, Balachandran C, Rao L. Zosteriform cutaneous metastases: A case report and brief review of literature. Indian J Dermatol Venereol Leprol 2010;76:447.
- Rendi MH, Dhar AD. Cutaneous metastasis of rectal adenocarcinoma. Dermatol Nurs 2003;15:131-2.
- Sarid D, Wigler N, Gutkin Z, Merimsky O, Leider-Trejo L, Ron IG. Cutaneous and subcutaneous metastases of rectal cancer. Int J Clin Oncol 2004;9:202-5.
- Hussein MR. Skin metastasis: A pathologist's perspective. J Cutan Pathol 2010;37:e1-20.
- Riley S, Wah T. Cutaneous metastasis of esophageal adenocarcinoma with an unusual presentation. J Clin Ultrasound 2007;35:289-92.
- Adyanthaya R. Multiple cutaneous metastases from esophageal adenocarcinoma. J Gastrointest Cancer 2008;39:22-5.
- Freeman CR, Rozenfeld M, Schopflocher P. Cutaneous metastases from carcinoma of the cervix. Arch Dermatol 1982;118:40-1.
- Brownstein MH, Helwig EB. Spread of tumors to the skin. Arch Dermatol 1973;107:80-6.

| Figure 1(a) Erythematous to hyperpigmented infiltrated nodules and plaques, showing peau dæorange appearance. (b) Close up view

| Figure 2(a) Low power: Dermis shows diffuse infiltration of tumor cells. Lymphoplasmacytic infiltrate around blood vessels. (b) High power: Dermal tumor cells are present in cords and singles, with pleomorphic round to oval nucleus, high N:C ratio, and vesicular chromatin. Signet ring-like cells are present

| Figure 3(a) Endoscopic view of gastroesophageal junction: Friable hypertrophic growth, which on biopsy showed features suggestive of poorly differentiated adenocarcinoma. (b) Endoscopic view of fundus of the stomach
References
- Rao R, Balachandran C, Rao L. Zosteriform cutaneous metastases: A case report and brief review of literature. Indian J Dermatol Venereol Leprol 2010;76:447.
- Rendi MH, Dhar AD. Cutaneous metastasis of rectal adenocarcinoma. Dermatol Nurs 2003;15:131-2.
- Sarid D, Wigler N, Gutkin Z, Merimsky O, Leider-Trejo L, Ron IG. Cutaneous and subcutaneous metastases of rectal cancer. Int J Clin Oncol 2004;9:202-5.
- Hussein MR. Skin metastasis: A pathologist's perspective. J Cutan Pathol 2010;37:e1-20.
- Riley S, Wah T. Cutaneous metastasis of esophageal adenocarcinoma with an unusual presentation. J Clin Ultrasound 2007;35:289-92.
- Adyanthaya R. Multiple cutaneous metastases from esophageal adenocarcinoma. J Gastrointest Cancer 2008;39:22-5.
- Freeman CR, Rozenfeld M, Schopflocher P. Cutaneous metastases from carcinoma of the cervix. Arch Dermatol 1982;118:40-1.
- Brownstein MH, Helwig EB. Spread of tumors to the skin. Arch Dermatol 1973;107:80-6.


 PDF
PDF  Views
Views  Share
Share

