A Case Report of Newly Diagnosed Epithelial Ovarian Carcinoma Presenting with Spontaneous Tumor Lysis Syndrome and Its Successful Management with Rasburicase
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(03): 360-362
DOI: DOI: 10.4103/ijmpo.ijmpo_23_16
Abstract
Tumor Lysis Syndrome (TLS) commonly occurs in hematological malignancies, but it is very rare in patients with a solid tumor. In cases of solid tumors, TLS usually occurs spontaneously or after the initiation of anticancer therapy, and it has a high mortality rate. This syndrome consists of a constellation of laboratory findings such as hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia known as laboratory TLS. When clinical complications such as seizures, acute renal failure, and cardiac dysrhythmias occur in patients with laboratory TLS, the syndrome is called clinical TLS. The present case report is sixth in the series and probably the first case report of spontaneous TLS in a newly diagnosed patient of epithelial ovarian cancer and also shows the effectiveness of single dose (1.5 mg) of rasburicase along with adequate hydration to rapidly reverse TLS and also timely initiation of definitive treatment. The patient was then able to complete successfully the planned neoadjuvant chemotherapy and surgery without any long-term sequela.
Publication History
Article published online:
04 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Tumor Lysis Syndrome (TLS) commonly occurs in hematological malignancies, but it is very rare in patients with a solid tumor. In cases of solid tumors, TLS usually occurs spontaneously or after the initiation of anticancer therapy, and it has a high mortality rate. This syndrome consists of a constellation of laboratory findings such as hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia known as laboratory TLS. When clinical complications such as seizures, acute renal failure, and cardiac dysrhythmias occur in patients with laboratory TLS, the syndrome is called clinical TLS. The present case report is sixth in the series and probably the first case report of spontaneous TLS in a newly diagnosed patient of epithelial ovarian cancer and also shows the effectiveness of single dose (1.5 mg) of rasburicase along with adequate hydration to rapidly reverse TLS and also timely initiation of definitive treatment. The patient was then able to complete successfully the planned neoadjuvant chemotherapy and surgery without any long-term sequela.
Introduction
Tumor lysis syndrome (TLS) is rare in solid tumors and particularly rare in epithelial ovarian cancer. It is unknown for carcinoma ovary to present with spontaneous clinical TLS. The management is similar to that in hematological malignancy, which includes adequate and aggressive hydration and use of allopurinol and rasburicase as add on drugs. Below mentioned case reports the successful management of spontaneous TLS in patient with newly diagnosed serous adenocarcinoma ovary.
Case Report
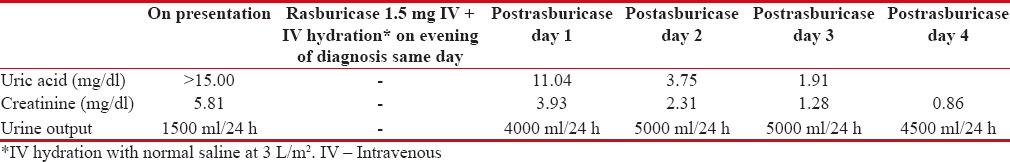
A 49-year-old premenopausal female presented with abdominal fullness and vomiting. She had a performance status-1, and clinical examination was suggestive of ascites and decreased air entry in right lower lobe. Blood investigation showed a creatinine of 5.81 mg/dl and uric acid >15 mg/dl. Her cancer antigen (CA)-125 was 3240 U/ml, carcinoembryonic antigen of 0.70 ng/ml, alpha-fetoprotein – 4.80 ng/ml, and beta human chorionic gonadotropin – 5.05 mIU/ml. Noncontrast computed tomography thorax and abdomen showed mild ascites, thickened infiltrated omentum forming omental caking, and the presence of moderate right pleural effusion. Both kidneys, ovary, uterus were normal. Pleural fluid cytology was suggestive of malignant cells. Fine needle aspiration cytology from omental cake was suggestive of metastatic adenocarcinoma. Diagnosis of stage IV peritoneal carcinomatosis/carcinoma ovary in clinical TLS with renal failure was made. She received a single dose of 1.5 mg rasburicase along with aggressive hydration at 3 L/m2. Urine output and daily creatinine and uric acid were monitored daily. The blood investigation on diagnosis and subsequent to diagnosis are mentioned in Table 1. A decision to start neoadjuvant chemotherapy (NACT) was made in view of advanced stage. Paclitaxel and carboplatin could be given postpresentation day 4 after reversal of TLS; however, hydration for 2 days' postchemotherapy and allopurinol for 7 days was given. She underwent cytoreductive surgery post three cycles NACT. Presurgery CA-125 was 24.20 U/ml, and contrast-enhanced computed tomography abdomen showed resolution of pleural effusion and partial response in omental deposits and no ascites. Her surgical pathology report showed marked response to chemotherapy with minimal residual poorly differentiated serous carcinoma involving left ovary and omentum and single pelvic lymph node. The patient then went on to complete three cycles of adjuvant chemotherapy. The patient is now apparently healthy, and her posttreatment ultrasonography abdomen pelvis and CA-125 are normal.
Table 1
Laboratory parameter at presentation and after rasburicase
|
Discussion
The most widely used diagnostic criteria for TLS were proposed by Cairo and Bishop in 2004.[1] Modifications to the 2004 Cairo–Bishop criteria, requiring two or more laboratory criteria within 24 h, and removing the definition of a 25% change in biochemical parameters for the definition of laboratory TLS, were proposed in 2011.[2] As the patient had acute kidney injury, it classifies into clinical TLS.
Based on a review article of TLS in solid tumors, so far, ten published reports of TLS in gynecological cancer have been reported.[3] In this series, six cases of ovarian cancer complicated with TLS were found. However, two cases were excluded as they were belonging to nonepithelial ovarian cancer histology.[4,5] All of the four cases of TLS were associated with chemotherapy.[3] The fifth case was published in 2015, and the present case report is sixth in the series and probably the first case report of spontaneous TLS in a newly diagnosed patient of epithelial ovarian cancer.
The TLS occurred after chemotherapy in all five cases. The first case reported in literature shows the development of TLS during induction chemotherapy with carboplatin and cyclophosphamide in a patient with serous ovarian adenocarcinoma.[6] A second case report describes TLS in a patient receiving salvage topotecan for recurrent serous ovarian cancer.[7] In both cases, the patients were treated using intravenous hydration and allopurinol. The third case reported after intravenous weekly paclitaxel for recurrent ovarian cancer with massive ascites and responded to treatment with a combination of vigorous intravenous hydration, furosemide, allopurinol, and sodium bicarbonate.[8] Fourth case report documents fatal TLS after receiving carboplatin and paclitaxel chemotherapy for recurrent ovarian cancer with documented intravascular spread.[9] The last case of TLS reported was after Docetaxel in patient with relapse serous carcinoma ovary and was successfully managed with Rasburicase.[10]
Intravenous hydration is a cornerstone of treatment to provide renal perfusion and increase urine output, which helps eliminate uric acid and phosphate. The exact volume of fluid required for hydration is not known, but it seems reasonable to aim for 3 lt/m2 of normal saline, with the aim of maintaining a urine output of >4 ml/Kg/hr in infants and 100 ml/m2/hr in adults. Alkalinization of the urine is not recommended in the treatment of TLS as both xanthine and hypoxanthine, that precede uric acid in the biochemical pathway, become less soluble in alkaline conditions and so precipitate before any uric acid can be formed thus negating any potential advantage of the increased solubility of uric acid at high urinary pH.[11]
Allopurinol is a xanthine oxidase inhibitor. It reduces the production of uric acid by decreasing the rate of the conversion of hypoxanthine to xanthine and xanthine to uric acid. Importantly, it does not increase the rate of breakdown of any uric acid that has already been formed and so its therapeutic effect is delayed by 24–72 h.[11] Rasburicase is a recombinant form of the enzyme urate oxidase which metabolizes urate to allantoin. It is extremely effective in reducing plasma levels of uric acid within 4 hours of administration, thus allowing early inititiation of chemotherapy. Given these respective mechanisms of action, where rasburicase is being used in the treatment or prophylaxis of TLS, the addition of allopurinol is unnecessary and has the potential to reduce the effectiveness of rasburicase. Although the licensed dose of rasburicase is 0.2 mg/kg/day, a number of publications have explored lower doses and shorter courses of therapy.[11]
While TLS remains a rare complication of the treatment of ovarian cancer, prompt recognition and treatment are essential to recovery and the avoidance of permanent renal injury. To the best of our knowledge, this is only second case of successful use of rasburicase in the management of TLS caused by ovarian malignancy.[10] Moreover, like in the previous case, only a single dose of 1.5 mg was successful in the management as compared to the higher doses recommended.[10] The patient is at present in complete remission on regular follow-up and after completing her adjuvant treatment and with normal renal parameters.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Cairo MS, Bishop M. Tumour lysis syndrome: New therapeutic strategies and classification. Br J Haematol 2004;127:3-11.
- Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. N Engl J Med 2011;364:1844-54
- Mirrakhimov AE, Ali AM, Khan M, Barbaryan A. Tumor lysis syndrome in solid tumors: An up to date review of the literature. Rare Tumors 2014;6:5389.
- Cui T, Wang C, He H, Shi G, Hu L. A rare case of ovarian Burkitt lymphoma associated tumor lysis syndrome. Eur J Gynaecol Oncol 2010;31:209-10.
- Doi M, Okamoto Y, Yamauchi M, Naitou H, Shinozaki K. Bleomycin-induced pulmonary fibrosis after tumor lysis syndrome in a case of advanced yolk sac tumor treated with bleomycin, etoposide and cisplatin (BEP) chemotherapy. Int J Clin Oncol 2012;17:528-31.
- Bilgrami SF, Fallon BG. Tumor lysis syndrome after combination chemotherapy for ovarian cancer. Med Pediatr Oncol 1993;21:521-4.
- Chan JK, Lin SS, McMeekin DS, Berman ML. Patients with malignancy requiring urgent therapy: CASE 3. Tumor lysis syndrome associated with chemotherapy in ovarian cancer. J Clin Oncol 2005;23:6794-5.
- Yahata T, Nishikawa N, Aoki Y, Tanaka K. Tumor lysis syndrome associated with weekly paclitaxel treatment in a case with ovarian cancer. Gynecol Oncol 2006;103:752-4.
- Camarata M, Davies R, Copley S, Blagden S. Tumour lysis syndrome in a patient with intravascular spread from a recurrent epithelial ovarian cancer. BMJ Case Rep 2013;2013. pii: Bcr2013009532.
- ;Roberts ME, DeSimone CP, Ueland FR, Baldwin LA. A case of tumor lysis syndrome after docetaxel administration for recurrent ovarian cancer. J Androl Gynaecol 2015;3:2.
- Jones GL, Will A, Jackson GH, Webb NJ, Rule S; British Committee for Standards in Haematology. Guidelines for the management of tumour lysis syndrome in adults and children with haematological malignancies on behalf of the British Committee for Standards in Haematology. Br J Haematol 2015;169:661-71.
References
- Cairo MS, Bishop M. Tumour lysis syndrome: New therapeutic strategies and classification. Br J Haematol 2004;127:3-11.
- Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. N Engl J Med 2011;364:1844-54
- Mirrakhimov AE, Ali AM, Khan M, Barbaryan A. Tumor lysis syndrome in solid tumors: An up to date review of the literature. Rare Tumors 2014;6:5389.
- Cui T, Wang C, He H, Shi G, Hu L. A rare case of ovarian Burkitt lymphoma associated tumor lysis syndrome. Eur J Gynaecol Oncol 2010;31:209-10.
- Doi M, Okamoto Y, Yamauchi M, Naitou H, Shinozaki K. Bleomycin-induced pulmonary fibrosis after tumor lysis syndrome in a case of advanced yolk sac tumor treated with bleomycin, etoposide and cisplatin (BEP) chemotherapy. Int J Clin Oncol 2012;17:528-31.
- Bilgrami SF, Fallon BG. Tumor lysis syndrome after combination chemotherapy for ovarian cancer. Med Pediatr Oncol 1993;21:521-4.
- Chan JK, Lin SS, McMeekin DS, Berman ML. Patients with malignancy requiring urgent therapy: CASE 3. Tumor lysis syndrome associated with chemotherapy in ovarian cancer. J Clin Oncol 2005;23:6794-5.
- Yahata T, Nishikawa N, Aoki Y, Tanaka K. Tumor lysis syndrome associated with weekly paclitaxel treatment in a case with ovarian cancer. Gynecol Oncol 2006;103:752-4.
- Camarata M, Davies R, Copley S, Blagden S. Tumour lysis syndrome in a patient with intravascular spread from a recurrent epithelial ovarian cancer. BMJ Case Rep 2013;2013. pii: Bcr2013009532.
- ;Roberts ME, DeSimone CP, Ueland FR, Baldwin LA. A case of tumor lysis syndrome after docetaxel administration for recurrent ovarian cancer. J Androl Gynaecol 2015;3:2.
- Jones GL, Will A, Jackson GH, Webb NJ, Rule S; British Committee for Standards in Haematology. Guidelines for the management of tumour lysis syndrome in adults and children with haematological malignancies on behalf of the British Committee for Standards in Haematology. Br J Haematol 2015;169:661-71.


 PDF
PDF  Views
Views  Share
Share

