A Case Report of Pleuropulmonary Blastoma Presenting as Tension Pneumothorax
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(01): 70-72
DOI: DOI: 10.4103/0971-5851.203515
Abstract
Pleuropulmonary blastoma (PPB) is a very rare, highly aggressive, and malignant tumor that originates from either lungs or pleura. It occurs mainly in children aged <5>Publication History
Article published online:
06 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Pleuropulmonary blastoma (PPB) is a very rare, highly aggressive, and malignant tumor that originates from either lungs or pleura. It occurs mainly in children aged <5>
Introduction
Primary pulmonary neoplasms are uncommon in children. One such tumor, pleuropulmonary blastoma (PPB), is very rare, highly aggressive and malignant, and originates from either the lungs or pleura.[1] It occurs mainly in children aged <5 href="https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5398112/#ref1" rid="ref1" class=" bibr popnode tag_hotlink tag_tooltip" id="__tag_603278597" role="button" aria-expanded="false" aria-haspopup="true" xss=removed>1,2] PPB was classified into three groups by Dehner et al. in 1995 as cystic (type I), mixed (type II), or solid (type III).[3] We describe a case of PPB which presented with unusual presentation of tension pneumothorax, with an aim to increase awareness so as to recognize this rare entity in early course.
Case Report
A 3-year-old female child was brought to the hospital with severe breathlessness which had been progressing over the last 2 months. The child also had complaints of weight loss and decreased oral intake for 1 month. Her previous medical and family history was unremarkable. On examination, the child had increased respiratory efforts with breath sounds diminished in the left lung zone.
Chest X-ray showed left side pneumothorax with mediastinal shift to opposite side [Figure 1].
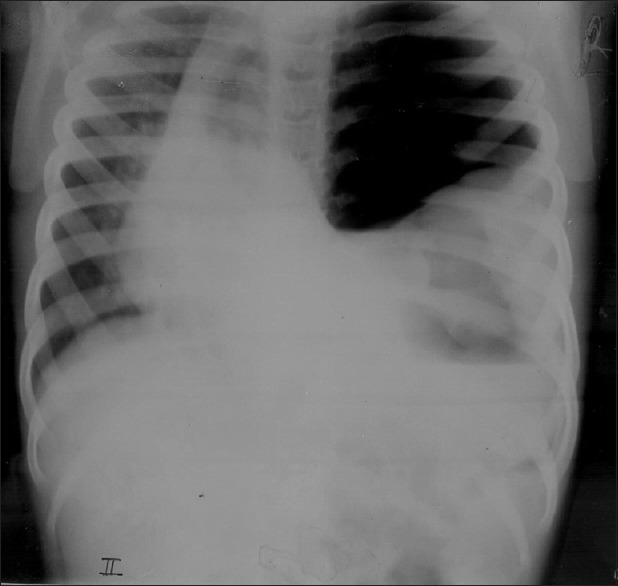
| Figure 1Massive pneumothorax
Intercostal drain was inserted immediately; child was intubated and mechanically ventilated and shifted to pediatric Intensive Care Unit.
Computerized tomography of thorax revealed a large mass in the left hemithorax (7 cm × 5 cm × 3 cm) containing solid mass [Figure 2]. Histological diagnosis of the tumor by ultrasonography (USG)-guided biopsy was PPB type III, [Figure 3], containing predominant solid components. Abdominal USG revealed no abnormality. Pediatric surgeon's opinion was taken and decided to start neoadjuvant chemotherapy as tumor was not amenable for resection.
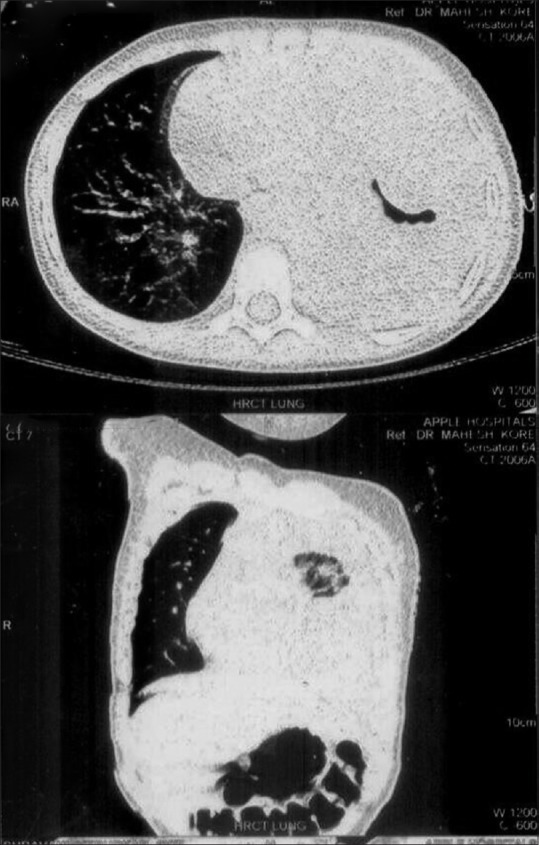
| Figure 2Computerized tomography chest – mass in the left hemithorax
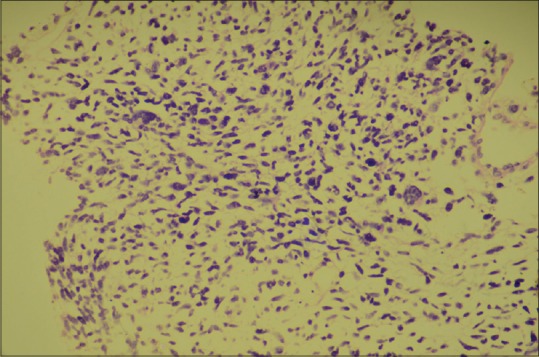
| Figure 3Biopsy specimen showing solid blastemal cells
Child was started on ifosfamide, vincristine, actinomycin D, and doxorubicin (IVADO) chemotherapy regimen.
After the completion of the first course of chemotherapy, dyspnea resolved, child was stable and discharged in an ambulatory state. Child was readmitted for the second course of chemotherapy 3 weeks after the first cycle of chemotherapy. However, she developed progressive respiratory distress. X-ray chest did not show any improvement in tumor size. Ultimately, patient succumbed to progressive disease after completion of the second cycle of chemotherapy.
Discussion
PPB is an aggressive tumor accounting for <1 href="https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5398112/#ref4" rid="ref4" class=" bibr popnode tag_hotlink tag_tooltip" id="__tag_603278592" role="button" aria-expanded="false" aria-haspopup="true" xss=removed>4] Morphologically, PPB has three types[5] (I, II, and III) and a fourth type (Ir) was added in 2006.[6] A progression from type I to type III may occur over time.[2] The age of presentation is usually <4 href="https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5398112/#ref7" rid="ref7" class=" bibr popnode" role="button" aria-expanded="false" aria-haspopup="true" xss=removed>7] Clinically, the patient may present with chest or upper abdominal pain, fever, dyspnea, cough, hemoptysis, anorexia, malaise, or neurological symptoms resulting from brains metastasis.[8]
Imaging studies show varied appearances such as unilocular cysts, a multicystic structure, a cyst containing a polypoid mass, and solid-cystic or entirely solid masses of variable sizes with or without the involvement of the pleura or chest wall and may fill the entire hemithorax. The entire thorax should be scrutinized for the presence of any asymptomatic cysts which could be forerunner of type I tumors.[9]
Child in our report presented with tension pneumothorax. The tumor has no characteristic findings on imaging studies; however, it should be considered in the differential diagnosis of other benign cystic lung lesions on imaging studies.[8]
Multiple needle core biopsies may be required as a single biopsy may not pick up the tumor. Immunohistochemical staining supports morphological diagnosis.
Three types of the PPB have been described based on morphology. Type I tumors carry the most favorable prognosis and account for 15%–20% of all PPB, whereas type II and type III tumors behave aggressively and together (distributed equally) account for 80%–85% of all PPB.[8]
Type I tumors are purely cystic, peripherally located, show striking absence of chest wall invasion, and may be single or multicystic and the cyst wall is usually thin without grossly visible solid areas.[9]
Type Ir (type I-regressed) tumors are cystic containing few spindle-shaped cells in the cyst wall with few foci of dystrophic calcification but without subepithelial malignant cell condensation. It might represent a regressed or a genetically destined but abortive type I tumor. Only 8% of such tumors show onward progression to PPB type II or type III. Its differential diagnosis is the same as for type I tumors.[9]
Type II tumors are partly solid and partly cystic, therefore sharing features of both type I and type III tumors. The cysts may be visible grossly or microscopically; if the cysts are seen only microscopically the tumors are called predominantly solid type II tumors, but if the tumor is largely cystic it is called predominantly cystic type II tumor.[9,10]
Type III tumors are entirely solid tumors usually presenting as large, well-circumscribed masses partly filling the hemithorax with or without attachment to the chest wall or mediastinum. The tumor may be friable due to hemorrhages and necrosis. There may be areas of infarction and necrosis affecting the cartilaginous, blastematous, or sarcomatous component producing pseudocysts.[9,10]
The most common site for metastatic spread is the brain.[7] The long-term survival of more than 80% has been reported for type I and <50 href="https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5398112/#ref7" rid="ref7" class=" bibr popnode" role="button" aria-expanded="false" aria-haspopup="true" xss=removed>7,9,10]
The recommended treatment for type I tumors consists of surgical excision and adjuvant chemotherapy. For type Ir tumors only follow-up but no chemotherapy is recommended. For the usual type II and type III tumors, the treatment consists of aggressive surgery and chemotherapy. For large type II and type III tumors, after initial confirmation by multiple needle core biopsies, 2–4 courses of neoadjuvant chemotherapy are instituted reducing the tumor size usually by more than 90%, followed by surgical resection. The recommended chemotherapeutic agents are IVADO regimen. For brain metastasis, the recommended treatment consists of all three modalities; that is surgery, radiation therapy, and chemotherapy in an attempt to achieve cure.[7,9]
Conclusion
- PPB is a distinct neoplasm with varied presentation which progress rapidly and altering outcomes
- Mandates early histological recognition and differentiation from other benign congenital malformations or cystic lesions and instituting early management accordingly.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Calabria R, Srikanth MS, Chamberlin K, Bloch J, Atkinson JB. Management of pulmonary blastoma in children. Am Surg 1993;59:192-6.
- Wright JR Jr. Pleuropulmonary blastoma: A case report documenting transition from type I (cystic) to type III (solid). Cancer 2000;88:2853-8.
- Dehner LP, Watterson J, Priest J. Pleuropulmonary blastoma: A unique intrathoracic pulmonary neoplasm of childhood. Perspect Pediatr Pathol 1995;18:214-26.
- Priest JR, Watterson J, Strong L, Huff V, Woods WG, Byrd RL, et al. Pleuropulmonary blastoma: A marker for familial disease. J Pediatr 1996;128:220-4.
- Khan AA, El-Borai AK, Alnoaiji M. Pleuropulmonary blastoma: A case report and review of the literature. Case Rep Pathol 2014;2014:509086.
- Priest JP. Pleuropulmonary blastoma. In: Rare Tumors in Children and Adolescents, Pediatr Oncology. Berlin: Springer; 2012. p. 213-21.
- Thomas Stocker J, Husain A, Dehner L. Pediatric Tumors. Dail and Hammar's Pulmonary Pathology. 2008;2(3):542-557.
- Priest JR, McDermott MB, Bhatia S, Watterson J, Manivel JC, Dehner LP. Pleuropulmonary blastoma: A clinicopathologic study of 50 cases. Cancer 1997;80:147-61.
- Weissferdt AMoran C. Malignant Biphasic Tumors of the Lungs. Advances in Anatomic Pathology. 2011;18(3):179-189.
- Home - The International Pleuropulmonary Blastoma Registry [Internet]. Ppbregistry.org. 2016. Available from: http://WWW.PPBREGISTRY.ORG. [Last cited on 2016 Nov 30].

| Figure 1Massive pneumothorax

| Figure 2Computerized tomography chest – mass in the left hemithorax

| Figure 3Biopsy specimen showing solid blastemal cells
References
- Calabria R, Srikanth MS, Chamberlin K, Bloch J, Atkinson JB. Management of pulmonary blastoma in children. Am Surg 1993;59:192-6.
- Wright JR Jr. Pleuropulmonary blastoma: A case report documenting transition from type I (cystic) to type III (solid). Cancer 2000;88:2853-8.
- Dehner LP, Watterson J, Priest J. Pleuropulmonary blastoma: A unique intrathoracic pulmonary neoplasm of childhood. Perspect Pediatr Pathol 1995;18:214-26.
- Priest JR, Watterson J, Strong L, Huff V, Woods WG, Byrd RL, et al. Pleuropulmonary blastoma: A marker for familial disease. J Pediatr 1996;128:220-4.
- Khan AA, El-Borai AK, Alnoaiji M. Pleuropulmonary blastoma: A case report and review of the literature. Case Rep Pathol 2014;2014:509086.
- Priest JP. Pleuropulmonary blastoma. In: Rare Tumors in Children and Adolescents, Pediatr Oncology. Berlin: Springer; 2012. p. 213-21.
- Thomas Stocker J, Husain A, Dehner L. Pediatric Tumors. Dail and Hammar's Pulmonary Pathology. 2008;2(3):542-557.
- Priest JR, McDermott MB, Bhatia S, Watterson J, Manivel JC, Dehner LP. Pleuropulmonary blastoma: A clinicopathologic study of 50 cases. Cancer 1997;80:147-61.
- Weissferdt AMoran C. Malignant Biphasic Tumors of the Lungs. Advances in Anatomic Pathology. 2011;18(3):179-189.
- Home - The International Pleuropulmonary Blastoma Registry [Internet]. Ppbregistry.org. 2016. Available from: http://WWW.PPBREGISTRY.ORG. [Last cited on 2016 Nov 30].


 PDF
PDF  Views
Views  Share
Share

