A Novel Translocation: t(2;14)(p12;q32) in a Case of Precursor B acute Lymphoblastic Leukemia
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(03): 407-408
DOI: DOI: 10.4103/ijmpo.ijmpo_172_16

|
Publication History
Article published online:
04 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Sir,
Precursor B-cell acute lymphoblastic leukemia (B-ALL) is a disease of cytogenetic heterogeneity characterized by aneuploidy and/or structural alterations in pediatric ALL.[1] Rearrangement of IgH gene is seen nearly in all cases of B-ALL, namely, t(8;14)(q24;q32) and its variants t(2;8)(p12;q24) and t(8;22)(q24;q11) with less frequency in precursor B-ALL.[2] Recent developments include identification of several novel IgH gene translocations and submicroscopic deletions,[3] the significance of which requires further study. We report here a novel translocation t(2;14)(p12;q32) in a case of pre-B-ALL with IgH gene rearrangement.
An 8-year-old male child presented with fever and body ache, persisting for a month; there was no history of bleeding manifestation or any other symptoms. On clinical examination, the child had cervical lymphadenopathy and mild hepatosplenomegaly. Hemogram revealed hemoglobin level of 11.9 g, a total count of 51.4 × 109/L, and a platelet count of 18 × 109/L. The peripheral blood film showed 85% blasts which were medium to large with scant cytoplasm, irregular nuclei, coarse chromatin, and 1–2 nucleoli. A bone marrow study revealed 80% blasts with similar morphology as in peripheral blood film [Figure 1]. Other marrow elements were suppressed. Bone marrow study suggested ALL of L2 morphology.
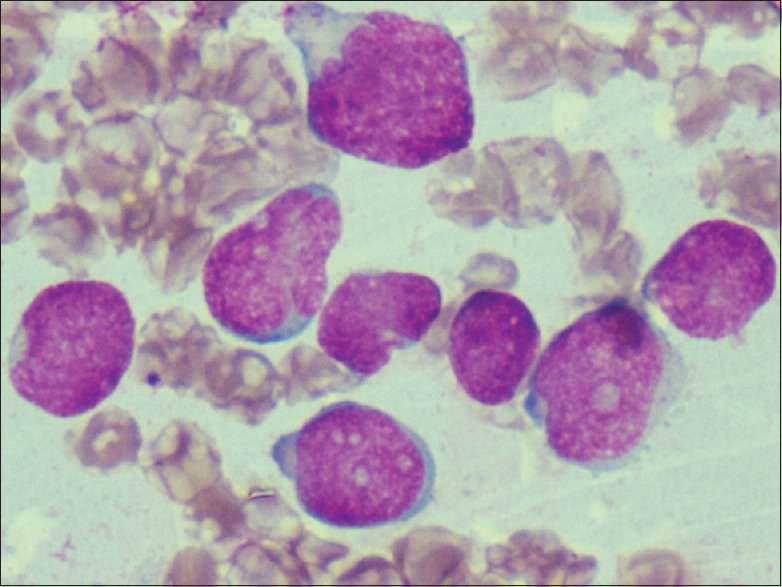
| Figure 1:Bone marrow film showing medium to large blasts with scant cytoplasm, irregular nuclei, coarse chromatin, and 1–2 nucleoli (Leishman's stain, ×400)
In flow cytometry study, CD45 gating was done and the CD45 dim positive blasts were found to be positive for terminal deoxynucleotidyl transferase, CD34, CD19, cCD79a, and CD10, with aberrant CD15 expression. Other myeloid and monocytic markers were negative. With bone marrow and flow cytometry studies, a diagnosis of precursor B-ALL was made, in accordance with the WHO 2008 classification.
Cytogenetic analysis was done on G-banded metaphases from unstimulated bone marrow short-term cultures. The karyotype was made in accordance with ISCN 2013.[4] Two abnormal clones were identified, one with the karyotype 47, XY, t(2;14)(p12;q32) +del (12)(p13) and another with 46, XY, t(2;14)(p12;q32) [Figure 2]. Here, the breakpoints noted were at 2p12 and 14q32, which are classically involved in Burkitt lymphoma ALL-L3. Hence, cytogenetics is in favor of ALL-L3. Due to disparity between hematological investigation and cytogenetics, fluorescence in situ hybridization (FISH) analysis was done using locus specific identifier IgH dual color break-apart rearrangement probe. 74.5% of cells showed the presence of rearrangement of IgH gene at 14q32 [Figure 3]. This finding confirms the involvement of 14q32 in t(2;14)(p12;q32) detected by routine cytogenetics. By considering all the findings, precursor B-ALL with IgH gene rearrangement was the final diagnosis made. The patient has been started on MCP-842 protocol including methotrexate, cyclophosphamide, and prednisolone and is currently in induction phase. There was no evidence of central nervous system involvement at the time of diagnosis.

| Figure 2:Idiogram showing 46, XY, t(2;14)(p12;q32) karyotype
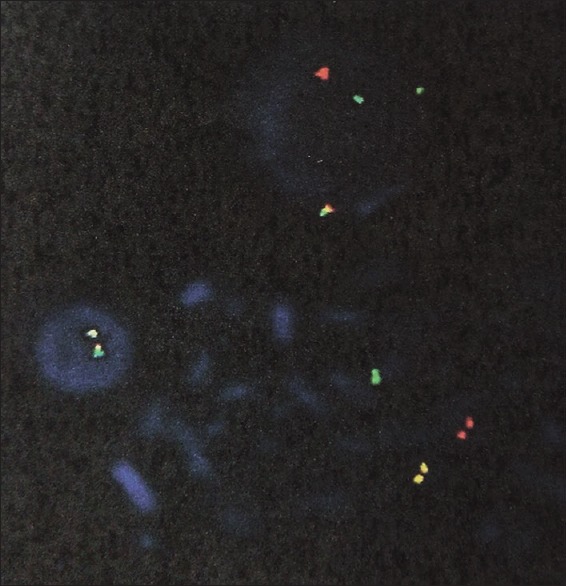
| Figure 3:Interphase fluorescence in situ hybridization using dual color break apart rearrangement probes showing rearrangement of IgH gene
Discussion
Precursor B-ALL is a neoplasm of precursor cells (lymphoblasts) committed to B-cell lineage. The 2008 WHO classification recognizes specific entities with unique genetic lesions and prognostic features. In addition, rare genetic alterations are reported and with advancement in molecular techniques, submicroscopic changes are increasingly being reported.[1,3]
IgH translocations in pre-B-ALL are very rare, constituting 2%–3%[3] and <5 href="https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5686998/#ref2" rid="ref2" class=" bibr popnode tag_hotlink tag_tooltip" id="__tag_649900238" role="button" aria-expanded="false" aria-haspopup="true" xss=removed>2] in other studies. These translocations have been detected with the use of break-apart rearrangement probe in interphase FISH. The most common translocation identified includes CRLF2/IgH, followed by inhibitor of DNA binding 4 and CCAAT enhancer-binding protein.[1] These IgH rearrangements are seen in older children and young adults with pre-B-ALL and they tend to have a poor prognosis[1] and may constitute subgroup of B-ALL.
A PubMed database search for t(2;14)(p12;q32) revealed two cases of pH +ve pre-B-ALL with t(2;14)(p13;q32) translocations;[5,6] other IgH translocations in pre-B-ALL reported include t(2;14)(q14.3;q32) and t(14;17)(q32;q21).[2] Three cases of de novo ALL with t(2;14)(p13;q32)[7] and three cases with the same translocation at relapse[8] have been reported in literature. The three cases reported by Watson et al.[7] showed variable immunophenotypes and complete remission at 14–19 months. Our patient is an 8-year-old male child with the sole abnormality of t(2;14)(p12;q32) which may be the first of its kind. Close follow-up will help understand the outcome of treatment in the future for this chromosomal abnormality. To the best of our knowledge, this is the first case of t(2;14)(p12;q32) with IgH rearrangement in a case of precursor B-ALL being reported.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Woo JS, Alberti MO, Tirado CA. Childhood B-acute lymphoblastic leukemia: A genetic update. Exp Hematol Oncol 2014;3:16.
- Dyer MJ, Akasaka T, Capasso M, Dusanjh P, Lee YF, Karran EL, et al. Immunoglobulin heavy chain locus chromosomal translocations in B-cell precursor acute lymphoblastic leukemia: Rare clinical curios or potent genetic drivers? Blood 2010;115:1490-9.
- Huh J, Mun YC, Yoo ES, Seong CM, Chung WS. Submicroscopic deletions of immunoglobulin heavy chain gene (IGH) in precursor B lymphoblastic leukemia with IGH rearrangements. Ann Lab Med 2015;35:128-31.
- Shaffer LG, McGowan-Jordan J, Schmid M. ISCN 2013: An International System for Human Cytogenetic Nomenclature. Basel: Karger Publishers; 2013.
- Inaba T, Oku N, Gotoh H, Murakami S, Oku N, Itoh K, et al. Philadelphia chromosome positive precursor B-cell acute lymphoblastic leukemia with a translocation t(2;14)(p13;q32). Leukemia 1991;5:719-22.
- Kerketta L, Vundinti BR, Ghosh K. Translocation t(2;14)(p13;q32) in a case of Ph+ acute lymphoblastic leukemia. Indian J Hum Genet 2007;13:125-6.
- Watson MS, Land VJ, Carroll AJ, Pullen J, Borowitz MJ, Link MP, et al. t(2;14)(p13;q32): A recurring abnormality in lymphocytic leukemia. A Pediatric Oncology Group study. Cancer Genet Cytogenet 1992;58:121-4.
- Berkowicz M, Toren A, Rosner E, Biniaminov M, Rosenthal E, Gipsh N, et al. Translocation (2;14)(p13;q32) in CD10+; CD13+ acute lymphatic leukemia. Cancer Genet Cytogenet 1995;83:140-3.Sir,

| Figure 1:Bone marrow film showing medium to large blasts with scant cytoplasm, irregular nuclei, coarse chromatin, and 1–2 nucleoli (Leishman's stain, ×400)

| Figure 2:Idiogram showing 46, XY, t(2;14)(p12;q32) karyotype

| Figure 3:Interphase fluorescence in situ hybridization using dual color break apart rearrangement probes showing rearrangement of IgH gene
References
- Woo JS, Alberti MO, Tirado CA. Childhood B-acute lymphoblastic leukemia: A genetic update. Exp Hematol Oncol 2014;3:16.
- Dyer MJ, Akasaka T, Capasso M, Dusanjh P, Lee YF, Karran EL, et al. Immunoglobulin heavy chain locus chromosomal translocations in B-cell precursor acute lymphoblastic leukemia: Rare clinical curios or potent genetic drivers? Blood 2010;115:1490-9.
- Huh J, Mun YC, Yoo ES, Seong CM, Chung WS. Submicroscopic deletions of immunoglobulin heavy chain gene (IGH) in precursor B lymphoblastic leukemia with IGH rearrangements. Ann Lab Med 2015;35:128-31.
- Shaffer LG, McGowan-Jordan J, Schmid M. ISCN 2013: An International System for Human Cytogenetic Nomenclature. Basel: Karger Publishers; 2013.
- Inaba T, Oku N, Gotoh H, Murakami S, Oku N, Itoh K, et al. Philadelphia chromosome positive precursor B-cell acute lymphoblastic leukemia with a translocation t(2;14)(p13;q32). Leukemia 1991;5:719-22.
- Kerketta L, Vundinti BR, Ghosh K. Translocation t(2;14)(p13;q32) in a case of Ph+ acute lymphoblastic leukemia. Indian J Hum Genet 2007;13:125-6.
- Watson MS, Land VJ, Carroll AJ, Pullen J, Borowitz MJ, Link MP, et al. t(2;14)(p13;q32): A recurring abnormality in lymphocytic leukemia. A Pediatric Oncology Group study. Cancer Genet Cytogenet 1992;58:121-4.
- Berkowicz M, Toren A, Rosner E, Biniaminov M, Rosenthal E, Gipsh N, et al. Translocation (2;14)(p13;q32) in CD10+; CD13+ acute lymphatic leukemia. Cancer Genet Cytogenet 1995;83:140-3.Sir,


 PDF
PDF  Views
Views  Share
Share

