A Prospective Study to Evaluate the Efficacy of the Fluorouracil, Leucovorin, Oxaliplatin, and Docetaxel Chemotherapy Regimen in Patients with Locally Advanced and Metastatic Adenocarcinoma of Stomach
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2022; 43(02): 153-158
DOI: DOI: 10.1055/s-0042-1742445
Abstract
Introduction In India, patients with gastric cancer present at an advanced stage, and there is no standard chemotherapy regimen. Al-Batran's fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT) chemotherapy gave us a glimmer of hope.
Objectives Hence, we intended to evaluate the efficacy of FLOT chemotherapy in locally advanced and metastatic adenocarcinoma of stomach.
Materials and Methods In this single-center, prospective cohort, patients with locally advanced and metastatic gastric adenocarcinoma who required chemotherapy between March 2016 and November 2017 were included in the study. All patients received standard FLOT chemotherapy. The primary objective was to evaluate the safety and efficacy of FLOT chemotherapy in the Indian population. Overall survival (OS) and progression-free survival (PFS) were calculated through the plotted Kaplan–Meier curves.
Results In our study, 28 patients received FLOT chemotherapy. Their mean age was 55 years (range, 28–70 years) with a male preponderance (89.3%). Twenty-five patients had metastatic disease (89.3%), and three had locally advanced disease (10.7%). The median number of cycles was 4.5 (range, 1–8), and 75%-received at least four cycles (n = 21). The hematological toxicities exhibited were neutropenia (50%) and febrile neutropenia (35.7%). Sixteen (57.1%) patients needed dose modifications due to treatment-related adverse effects (AEs). AEs led to treatment discontinuation in seven (25%) patients after the first cycle. The overall response rate in the intent-to-treat population was 52.7%, with the best-obtained response being a partial response, median PFS of 5 months, and median OS of 13 months.
Conclusion FLOT chemotherapy regimens induced excellent responses but with significantly increased toxicity, needing dose modifications, and hence, should be considered only in a young and fit patient.
Keywords
adenocarcinoma of the stomach - fluorouracil - leucovorin - oxaliplatin - docetaxel chemotherapy - locally advanced - metastaticFinancial Support and Sponsorship
None.
Publication History
Article published online:
08 March 2022
© 2022. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Introduction In India, patients with gastric cancer present at an advanced stage, and there is no standard chemotherapy regimen. Al-Batran's fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT) chemotherapy gave us a glimmer of hope.
Objectives Hence, we intended to evaluate the efficacy of FLOT chemotherapy in locally advanced and metastatic adenocarcinoma of stomach.
Materials and Methods In this single-center, prospective cohort, patients with locally advanced and metastatic gastric adenocarcinoma who required chemotherapy between March 2016 and November 2017 were included in the study. All patients received standard FLOT chemotherapy. The primary objective was to evaluate the safety and efficacy of FLOT chemotherapy in the Indian population. Overall survival (OS) and progression-free survival (PFS) were calculated through the plotted Kaplan–Meier curves.
Results In our study, 28 patients received FLOT chemotherapy. Their mean age was 55 years (range, 28–70 years) with a male preponderance (89.3%). Twenty-five patients had metastatic disease (89.3%), and three had locally advanced disease (10.7%). The median number of cycles was 4.5 (range, 1–8), and 75% received at least four cycles (n = 21). The hematological toxicities exhibited were neutropenia (50%) and febrile neutropenia (35.7%). Sixteen (57.1%) patients needed dose modifications due to treatment-related adverse effects (AEs). AEs led to treatment discontinuation in seven (25%) patients after the first cycle. The overall response rate in the intent-to-treat population was 52.7%, with the best-obtained response being a partial response, median PFS of 5 months, and median OS of 13 months.
Conclusion FLOT chemotherapy regimens induced excellent responses but with significantly increased toxicity, needing dose modifications, and hence, should be considered only in a young and fit patient.
Keywords
adenocarcinoma of the stomach - fluorouracil - leucovorin - oxaliplatin - docetaxel chemotherapy - locally advanced - metastaticIntroduction
Gastric cancer is the fifth most common cancer among males and the seventh among females in India. It is also the second most common cause of death globally.[1] [2]
In India, the age-adjusted rate (AAR) of gastric cancer is 3.0 to 13.2, whereas the global AAR for gastric cancer ranges between 4.1 and 95.5.[3] [4] [5] [6]
Indian patients are usually locally advanced or metastatic at presentation. As weight loss and loss of appetite are a significant concern, treatment with aggressive regimens at full dose to extract maximum benefit from chemotherapy becomes an uphill task. Optimization of a standard regimen to improve survival with minimal toxicities is the need of the hour in gastric cancers.
There is no universal standard chemotherapy regimen in first-line treatment for locally advanced or metastatic adenocarcinoma, and the prognosis is still abysmal with a median survival of 6 to 10 months despite treatment with combination chemotherapy, but for modified docetaxel, cisplatin, and fluorouracil (DCF) regimen by Manish Shah et al documented a median progression-free survival (PFS) and overall survival (OS) of 9.7 and 18.8 months, respectively, in a randomized Phase II study with lesser toxicities in comparison with the standard DCF regimen; however, randomized Phase III trials have shown that combination chemotherapy improved survival and quality of life compared with best supportive care.[7]
In the early days, two-drug combination of fluorouracil (FU) and cisplatin-containing combinations was considered standard therapy for patients with advanced gastric cancer in terms of response rate with no survival benefit.[8] Later, there was evidence that showed that the addition of DCF was superior to cisplatin and FU alone (CF) in terms of quality of life, response rate, time to progression, and OS.[9] [10] [11] Despite these benefits, standard DCF is criticized and not preferred due to its toxicity profile but a modified DCF triplet regimen was considered the standard of care in the first-line treatment of locally advanced and metastatic adenocarcinoma of the stomach.[12]
Several oxaliplatin-based regimens have been evaluated for gastric cancer,[13] [14] [15] [16] with the most intensively investigated regimen being a biweekly (once every 2 weeks) combination of infusional 5-FU (24 hour), leucovorin, and oxaliplatin (FLO).[17] [18] [19] This regimen (FLO) has fewer toxicities and thromboembolic events when compared with that of the cisplatin-based regimen.[13] The results of FLO regimen raised the interest of using this regimen with docetaxel instead of the classical CF regimen.[20]
In 2008, Al-Batran, in a Phase II trial with FU, leucovorin, oxaliplatin, and docetaxel (FLOT) chemotherapy in metastatic gastric cancer, showed a significant response rate of 57.7%, with a median PFS of 5.2 months and an OS of 11.1 months in the Western population. The quadruple FLOT regimen has been the new standard of care for locally advanced and resectable gastric adenocarcinoma after the FLOT-4 study. Indian gastric cancers are extremely cachexic with poor oral intake at the time of diagnosis and have varied tolerance with increased toxicities while treated with standard DCF regimen. Using lower doses of docetaxel and replacing cisplatin with oxaliplatin in FLOT compared with standard DCF was hypothesized to improve survival outcomes with a better toxicity profile; hence, this study was taken up to assess the safety and efficacy of FLOT chemotherapy with 5-fluorouracil, leucovorin, oxaliplatin, and docetaxel in our patient population.
Materials and Methods
Study Design
This is a single-center, prospective study of patients with locally advanced and metastatic gastric adenocarcinoma who required chemotherapy, treated at Amrita Institute of Medical Sciences, Kochi, between March 2016 and November 2017.
Inclusion Criteria
-
Those with histologically confirmed or biopsy-proven adenocarcinoma of the stomach who receive chemotherapy with FLOT regimen
-
Those with age >18 years
-
Those with Eastern Cooperative Oncology Group[21] PS 2 or less
-
Those with no history of synchronous or double malignancy
-
Those in locally advanced gastric cancer (defined as clinical stages T3N1 or T4N1 as determined by computed tomography [CT] scans and endoscopic ultrasonography)
-
Patients willing to abide and sign an informed consent.
Exclusion Criteria
-
Patients with proven preexisting peripheral neuropathy.
-
Those with brain metastasis.
-
Those with cancers arising from the gastroesophageal junction and squamous cell histology.
-
Those with cardiac dysfunction, human immunodeficiency virus-positive patients, or those with other immunodeficiency syndromes
-
Those with a history of hypersensitivity to FLOT chemotherapeutic drugs.
The FLOT four-drug regimen constitutes 5-fluorouracil, leucovorin, oxaliplatin, and docetaxel. The dose of each drug was as follows: 5-FU at 1200 mg/m2/day as a 24-hour infusion on days 1 and 2, docetaxel at 50 mg/m2, and oxaliplatin at 85 mg/m2 each as a 2-hour intravenous (IV) infusion on day 1. Injection leucovorin at 400 mg/m2 was administered as IV infusion on days 1 and 2. The drugs were cycled every 2 weeks or once in 14 days, and up to a total of eight cycles were given for patients with metastatic disease. In locally advanced gastric adenocarcinoma, four cycles of neoadjuvant (preoperative) chemotherapy followed by surgery and four more cycles of adjuvant (postoperative) chemotherapy were planned. Reassessment was performed at the completion of four cycles of FLOT, and toxicities were noted at the end of every cycle.
Standard antiemetic prophylaxis was done as per institution protocols. Prophylactic dexamethasone 8 mg was administered (days: 0–2) to prevent fluid retention and allergic reactions. Three doses of prophylactic growth factors (granulocyte colony-stimulating factor or Grafeel 300 µg subcutaneous once daily for 3 days) from day 3 of chemotherapy after each cycle were permitted. In patients with Grade IV toxicities or febrile neutropenia, 50% dose reduction was permitted with increase in use or prophylactic growth factor.
Before treatment, a complete general physical examination along with past medical history, complete blood count, blood chemistry, and pretreatment CT scans of chest, abdomen, and pelvis (CAP) was done 3 weeks prior to start of the treatment.
Toxicity was evaluated before the start of each cycle as per the National Cancer Institute - Common Toxicity Criteria (NCI-CTC) version 3.0.
For every four cycles, the objective response was evaluated as per the Response evaluation criteria in solid tumors (RECIST) criteria v1.1 based on a CT-scan of CAP (magnetic resonance imaging, when indicated) and they were compared with a baseline CT scan.
In the locally advanced setting, patients received four cycles of neoadjuvant chemotherapy followed by four more cycles after surgery and in the metastatic setting, a maximum of eight cycles of biweekly chemotherapy were planned. The primary objective was to evaluate the safety and efficacy of the regimen, whereas the secondary objective was OS and PFS.
Statistical Analysis
The data were analyzed using IBM SPSS software (Version 20.0 for Microsoft). With FLOT regimen, the efficacy was calculated using the percentage of cases with response to treatment.
Kaplan–Meier survival curves were plotted to measure the OS rates and PFS rates.
OS is defined as the time from randomization to death from any cause.
PFS is defined as the time from randomization until the first evidence of tumor progression or until death from any cause, whichever comes first.
Ethics
The procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional) and with the Helsinki Declaration of 1964, as revised in 2013. Ethical committee approval was obtained from Amrita Institute of Medical Sciences Ethics Committee, IEC-AIMS-2017-MEDONCO-472 on 26–12–2017. Informed patient consent was obtained prior to enrolment of participants.
Results
A total of 28 patients were treated with the FLOT chemotherapy regimen. Their mean age was 55.7 ± 9.8 years (range, 28–70 years), and there was a male preponderance (89.3%). [Table 1] depicts the demographics of patients with gastric adenocarcinoma included in the study.
|
General characteristics |
Number of patients, n (%) |
|---|---|
|
Stage |
|
|
III |
3 (10.7) |
|
IV |
25 (89.3) |
|
Sex distribution |
|
|
Male |
25 (89.3) |
|
Female |
3 (10.7) |
|
Lauren classification |
|
|
Diffuse |
14 (50.0) |
|
Intestinal |
14 (50.0) |
|
Prior treatment |
n (%) |
|---|---|
|
Surgery |
6 (21.4) |
|
Perioperative chemotherapy |
6 (21.4) |
|
Radiation |
1 (3.5) |
|
No prior treatments |
22 (78.5) |
|
Site of metastasis |
Number of patients, n (%)* |
|---|---|
|
Liver |
9 (32.1) |
|
Colon |
6 (21.2) |
|
Peritoneum with ascites |
4 (14.2) |
|
Omentum |
4 (14.2) |
|
Peritoneum |
2 (7.1) |
|
Lung |
1 (3.5) |
|
Ovary |
1 (3.5) |
|
Bone |
1 (3.5) |
|
Adrenal |
1 (3.5) |
|
Skin |
1 (3.5) |
|
Toxicity |
Grade |
Number of patients |
Total, n (%) |
|---|---|---|---|
|
Mucositis |
2 |
0 |
10 (35.7) |
|
3 |
9 |
||
|
4 |
1 |
||
|
Peripheral neuropathy |
2 |
4 |
9 (32.1) |
|
3 |
4 |
||
|
4 |
1 |
||
|
Diarrhea |
2 |
2 |
8 (28.5) |
|
3 |
6 |
||
|
4 |
6 |
||
|
Fatigue |
2 |
0 |
10 (35.7) |
|
3 |
6 |
||
|
4 |
4 |
|
Responses obtained |
Number of patients, n (%) |
|---|---|
|
CR |
0 |
|
PR |
11 (39.2) |
|
ORR = CR + PR |
11 (39.2) on ITT analysis, ORR = (52.3) |
|
Progression |
10 (35.7) |
|
Stable disease |
2 (7.1) |
|
Response not assessed |
5 (17.8) |
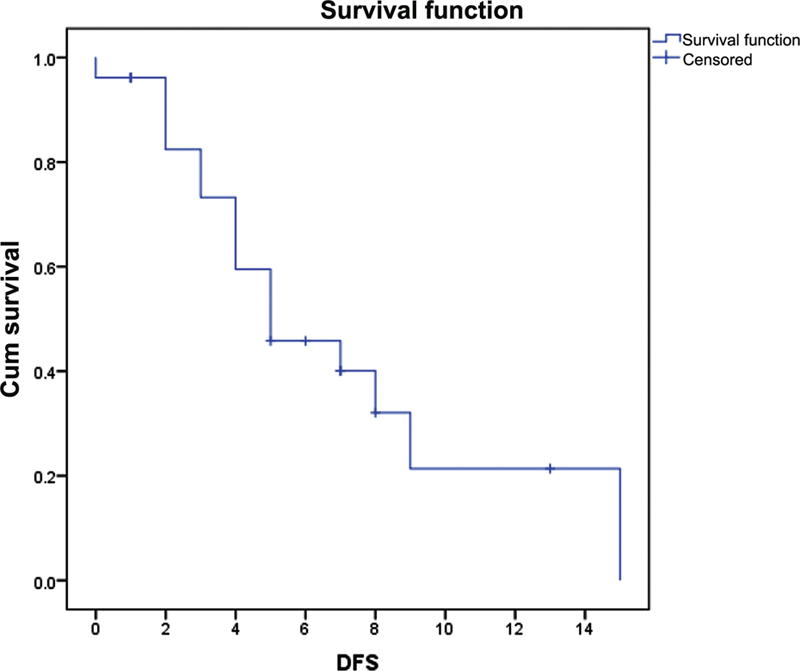
| Fig. 1 The disease-free survival (DFS) for the study group was 5 months (95%-confidence interval: 1.78–8.21).
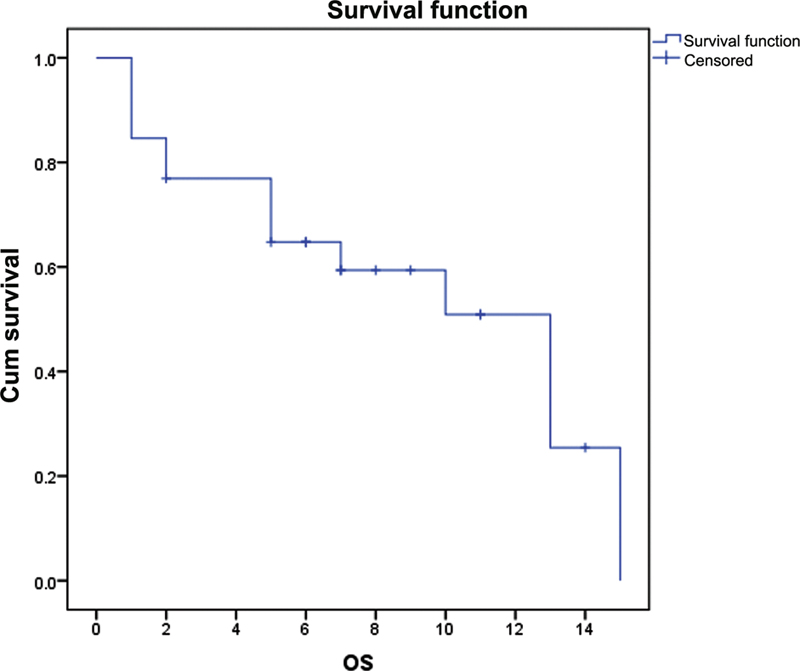
| Fig. 2The overall survival (OS) for fluorouracil, leucovorin, oxaliplatin, and docetaxel chemotherapy was 13 months (95%-confidence interval: 8.14–17.85).
Discussion
The biology of gastric cancer is extremely unique and aggressive by behavior, with most of them being either metastatic or locally advanced and inoperable at the time of diagnosis. Despite all efforts in oncology, improving survival and quality of life has been challenging in gastric adenocarcinoma. Platinum, taxane, and fluoroquinolone compounds in various two or three drug combinations have been the mainstay of treatment in gastric adenocarcinoma since the early 90s in both locally advanced and metastatic scenarios. Treating gastric adenocarcinoma with the right combination at the optimal therapeutic dose with tolerable side effects might most likely improve responses that translate into a survival benefit.
In our study, we intended to assess the safety and tolerability of the FLOT regimen. On ITT analysis, the overall response rate was 52.3%, with PR being the best-obtained response. The median time to progression was 5 months and the median OS was 13 months with significantly increased adverse events and more than half of the patients in the study needed dose modifications.
The response rates were very similar to that of the reported literature, with most of the regimens being active in gastric cancer such as ECF, XP, FOLFOX, or EOX, at a rate of ∼45% ± 10%.[9]
In the landmark study, REAL-2, the median OS to ECF, XP, FOLFOX, or EOX was between 9 and 13.3 months, with a 13-month OS with FLOT regimen in our study. The reason for no significant improvement in median OS in our patients with gastric cancer is probably multifactorial, as the majority of our patients are emaciated, with low socioeconomic status and had a significant weight loss either due to advanced disease (cancer cachexia per se) or low food intake with an average weight of patients in our study being 53 kg with a mean body surface area of 1.525.
There was a significant increase in hematological and nonhematological adverse events with Grades III to IV neutropenia in more than half of patients despite receiving three doses of prophylactic growth factors with every cycle. Higher grades of febrile neutropenia were seen in one-third of the patients (35.7%) with FLOT regimen. Increased all-grade gastrointestinal toxicity in the form of severe diarrhea and vomiting was documented in 21.4 and 10.7%-of the patients, respectively. Similarly, increased hematological and gastrointestinal adverse events were reported with three-drug DCF regimen in the V325 study and Al-Batrans' 2008 FLOT study with 48%-Grades III to IV neutropenia and 14.8%-Grades III to IV diarrhea.
More than half (57.1%) of the patients needed dose modifications due to chemotherapy-related adverse effects, with over a quarter of the patients stopping further chemotherapy after one cycle due to various reasons, mostly due to toxicity-related discontinuation.
The neurotoxicity related to oxaliplatin and docetaxel is well known and is the primary reason for stopping the chemotherapy. As both drugs cause peripheral neuropathy, we were cautious about combining them, but surprisingly, neurotoxicity was not very high with this regimen—the 17.8% of Grade III to IV peripheral neuropathy in our study was similar to the 17%-reported in the GATE study.[22]
Within the limitations of small sample size, FLOT chemotherapy regimen has had some activity in linitis plastica, which is relatively chemoresistant,[23] and among 13 patients with diffuse gastric cancer, we observed four PRs corresponding to a response rate of 30.7%. The best response documented in diffuse gastric carcinoma with signet ring cell morphology was a PR.
Four patients had extensive omental deposits with ascites at the time of presentation; after initiating FLOT chemotherapy, ascites disappeared after three cycles in three patients (75%), which suggests that it is an active regimen in extensive peritoneal disease as well.
Our patients with gastric adenocarcinoma seem to have very poor tolerance to the FLOT chemotherapy regimen with significant toxicities; hence, alternative or modified regimens need to be explored to improve outcomes. The need of hospitalization for 2 days and peripheral venous access such as a peripherally inserted central catheter line to administer chemotherapy is also a major challenge as many a time the cost of treatment has to be borne by the patient himself/herself from his/her own expenditure with no health insurance. Hence, quadruple FLOT regimen should be considered only in a patient with good performance status and no significantly associated comorbidities.
Conclusion
FLOT chemotherapy regimens induced excellent responses in locally advanced and metastatic adenocarcinoma of the stomach but with significantly increased adverse events, needing dose modifications in nearly two-thirds of the patients and hence, it should be considered only in a young patient with good performance status and no associated comorbidities.
Conflict of Interest
None declared.
Financial Support and Sponsorship
None.
References
- Rao DN, Ganesh B. Estimate of cancer incidence in India in 1991. Indian J Cancer 1998; 35 (01) 10-18
- Alberts SR, Cervantes A, van de Velde CJ. Gastric cancer: epidemiology, pathology and treatment. Ann Oncol 2003; 14 (Suppl. 02) ii31-ii36
- Pavithran K, Doval DC, Pandey KK. Gastric cancer in India. Gastric Cancer 2002; 5 (04) 240-243
- Yeole BB. Trends in cancer incidence in esophagus, stomach, colon, rectum and liver in males in India. Asian Pac J Cancer Prev 2008; 9 (01) 97-100
- Satyanarayana L, Asthana S. Life time risk for development of ten major cancers in India and its trends over the years 1982 to 2000. Indian J Med Sci 2008; 62 (02) 35-44
- Rastogi T, Devesa S, Mangtani P. et al. Cancer incidence rates among South Asians in four geographic regions: India, Singapore, UK and US. Int J Epidemiol 2008; 37 (01) 147-160
- Ajani JA. Evolving chemotherapy for advanced gastric cancer. Oncologist 2005; 10 (Suppl. 03) 49-58
- Foukakis T, Lundell L, Gubanski M, Lind PA. Advances in the treatment of patients with gastric adenocarcinoma. Acta Oncol 2007; 46 (03) 277-285
- Van Cutsem E, Moiseyenko VM, Tjulandin S. et al; V325 Study Group. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 2006; 24 (31) 4991-4997
- Ajani JA, Moiseyenko VM, Tjulandin S. et al; V-325 Study Group. Quality of life with docetaxel plus cisplatin and fluorouracil compared with cisplatin and fluorouracil from a phase III trial for advanced gastric or gastroesophageal adenocarcinoma: the V-325 Study Group. J Clin Oncol 2007; 25 (22) 3210-3216
- Ajani JA, Moiseyenko VM, Tjulandin S. et al; V-325 Study Group. Clinical benefit with docetaxel plus fluorouracil and cisplatin compared with cisplatin and fluorouracil in a phase III trial of advanced gastric or gastroesophageal cancer adenocarcinoma: the V-325 Study Group. J Clin Oncol 2007; 25 (22) 3205-3209
- Shah MA, Janjigian YY, Stoller R. et al. Randomized multicenter phase ii study of modified docetaxel, cisplatin, and fluorouracil (DCF) Versus DCF Plus growth factor support in patients with metastatic gastric adenocarcinoma: A study of the US gastric cancer consortium. J Clin Oncol 2015; 33 (33) 3874-3879
- Hejna M, Raderer M, Zacherl J. et al. Phase II study of docetaxel in combination with oxaliplatin in patients with metastatic or locally advanced esophagogastric cancer previously untreated with chemotherapy for advanced disease: results of the Central European Cooperative Oncology Group Study ESGAS.1.2.001. Anticancer Drugs 2008; 19 (05) 535-539
- Kim JG, Sohn SK, Chae YS. et al. Multicenter phase II study of docetaxel plus oxaliplatin combination chemotherapy in patients with advanced gastric cancer: Daegu Gyeongbuk Oncology Group. Br J Cancer 2008; 98 (03) 542-546
- Park Y, Kim K, Choi M. et al. A Phase I/II trial of docetaxel (D) and oxaliplatin (O) in patients with advanced gastric cancer (AGC). In: 2008 ASCO annual meeting proceedings (post-meeting edition). J Clin Oncol 2008; 26: 15S
- Richards D, McCollum D, Wilfong L. et al. Phase II trial of docetaxel and oxaliplatin in patients with advanced gastric cancer and/or adenocarcinoma of the gastroesophageal junction. Ann Oncol 2008; 19 (01) 104-108
- Al-Batran SE, Hartmann JT, Probst S. et al; Arbeitsgemeinschaft Internistische Onkologie. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol 2008; 26 (09) 1435-1442
- Al-Batran SE, Atmaca A, Hegewisch-Becker S. et al. Phase II trial of biweekly infusional fluorouracil, folinic acid, and oxaliplatin in patients with advanced gastric cancer. J Clin Oncol 2004; 22 (04) 658-663
- Al-Batran SE, Kerber A, Atmaca A. et al. Mitomycin C, 5-fluorouracil, leucovorin, and oxaliplatin as a salvage therapy for patients with cisplatin-resistant advanced gastric cancer: a phase I dose escalation trial. Onkologie 2007; 30 (1-2): 29-34
- Al Batron S, Hartman J, Hofheinz R. et al. Biweekly fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT) for patients with metastatic adenocarcinoma of the stomach or esophagogastric junction: A Phase II trial of the Arbeitsgemein schaft internistische Onkologie. Ann Oncol 2006; 19: 1882-1887
- Oken MM, Creech RH, Tormey DC. et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982; 5 (06) 649-655
- Cutsem VE, Boni C, Tabernero J. et al. Randomized Phase II study (GATE study) of docetaxel plus oxaliplatin with or without fluorouracil or capecitabine in metastatic or locally recurrent gastric cancer. J Clin Oncol 2011; (Suppl): 4018
- Messager M, Lefevre JH, Pichot-Delahaye V, Souadka A, Piessen G, Mariette C. FREGAT working group - FRENCH. The impact of perioperative chemotherapy on survival in patients with gastric signet ring cell adenocarcinoma: a multicenter comparative study. Ann Surg 2011; 254 (05) 684-693 , discussion 693
Address for correspondence
Publication History
Article published online:
08 March 2022
© 2022. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2,
Noida-201301 UP, India

| Fig. 1 The disease-free survival (DFS) for the study group was 5 months (95% confidence interval: 1.78–8.21).

| Fig. 2The overall survival (OS) for fluorouracil, leucovorin, oxaliplatin, and docetaxel chemotherapy was 13 months (95% confidence interval: 8.14–17.85).
References
- Rao DN, Ganesh B. Estimate of cancer incidence in India in 1991. Indian J Cancer 1998; 35 (01) 10-18
- Alberts SR, Cervantes A, van de Velde CJ. Gastric cancer: epidemiology, pathology and treatment. Ann Oncol 2003; 14 (Suppl. 02) ii31-ii36
- Pavithran K, Doval DC, Pandey KK. Gastric cancer in India. Gastric Cancer 2002; 5 (04) 240-243
- Yeole BB. Trends in cancer incidence in esophagus, stomach, colon, rectum and liver in males in India. Asian Pac J Cancer Prev 2008; 9 (01) 97-100
- Satyanarayana L, Asthana S. Life time risk for development of ten major cancers in India and its trends over the years 1982 to 2000. Indian J Med Sci 2008; 62 (02) 35-44
- Rastogi T, Devesa S, Mangtani P. et al. Cancer incidence rates among South Asians in four geographic regions: India, Singapore, UK and US. Int J Epidemiol 2008; 37 (01) 147-160
- Ajani JA. Evolving chemotherapy for advanced gastric cancer. Oncologist 2005; 10 (Suppl. 03) 49-58
- Foukakis T, Lundell L, Gubanski M, Lind PA. Advances in the treatment of patients with gastric adenocarcinoma. Acta Oncol 2007; 46 (03) 277-285
- Van Cutsem E, Moiseyenko VM, Tjulandin S. et al; V325 Study Group. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 2006; 24 (31) 4991-4997
- Ajani JA, Moiseyenko VM, Tjulandin S. et al; V-325 Study Group. Quality of life with docetaxel plus cisplatin and fluorouracil compared with cisplatin and fluorouracil from a phase III trial for advanced gastric or gastroesophageal adenocarcinoma: the V-325 Study Group. J Clin Oncol 2007; 25 (22) 3210-3216
- Ajani JA, Moiseyenko VM, Tjulandin S. et al; V-325 Study Group. Clinical benefit with docetaxel plus fluorouracil and cisplatin compared with cisplatin and fluorouracil in a phase III trial of advanced gastric or gastroesophageal cancer adenocarcinoma: the V-325 Study Group. J Clin Oncol 2007; 25 (22) 3205-3209
- Shah MA, Janjigian YY, Stoller R. et al. Randomized multicenter phase ii study of modified docetaxel, cisplatin, and fluorouracil (DCF) Versus DCF Plus growth factor support in patients with metastatic gastric adenocarcinoma: A study of the US gastric cancer consortium. J Clin Oncol 2015; 33 (33) 3874-3879
- Hejna M, Raderer M, Zacherl J. et al. Phase II study of docetaxel in combination with oxaliplatin in patients with metastatic or locally advanced esophagogastric cancer previously untreated with chemotherapy for advanced disease: results of the Central European Cooperative Oncology Group Study ESGAS.1.2.001. Anticancer Drugs 2008; 19 (05) 535-539
- Kim JG, Sohn SK, Chae YS. et al. Multicenter phase II study of docetaxel plus oxaliplatin combination chemotherapy in patients with advanced gastric cancer: Daegu Gyeongbuk Oncology Group. Br J Cancer 2008; 98 (03) 542-546
- Park Y, Kim K, Choi M. et al. A Phase I/II trial of docetaxel (D) and oxaliplatin (O) in patients with advanced gastric cancer (AGC). In: 2008 ASCO annual meeting proceedings (post-meeting edition). J Clin Oncol 2008; 26: 15S
- Richards D, McCollum D, Wilfong L. et al. Phase II trial of docetaxel and oxaliplatin in patients with advanced gastric cancer and/or adenocarcinoma of the gastroesophageal junction. Ann Oncol 2008; 19 (01) 104-108
- Al-Batran SE, Hartmann JT, Probst S. et al; Arbeitsgemeinschaft Internistische Onkologie. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol 2008; 26 (09) 1435-1442
- Al-Batran SE, Atmaca A, Hegewisch-Becker S. et al. Phase II trial of biweekly infusional fluorouracil, folinic acid, and oxaliplatin in patients with advanced gastric cancer. J Clin Oncol 2004; 22 (04) 658-663
- Al-Batran SE, Kerber A, Atmaca A. et al. Mitomycin C, 5-fluorouracil, leucovorin, and oxaliplatin as a salvage therapy for patients with cisplatin-resistant advanced gastric cancer: a phase I dose escalation trial. Onkologie 2007; 30 (1-2): 29-34
- Al Batron S, Hartman J, Hofheinz R. et al. Biweekly fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT) for patients with metastatic adenocarcinoma of the stomach or esophagogastric junction: A Phase II trial of the Arbeitsgemein schaft internistische Onkologie. Ann Oncol 2006; 19: 1882-1887
- Oken MM, Creech RH, Tormey DC. et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982; 5 (06) 649-655
- Cutsem VE, Boni C, Tabernero J. et al. Randomized Phase II study (GATE study) of docetaxel plus oxaliplatin with or without fluorouracil or capecitabine in metastatic or locally recurrent gastric cancer. J Clin Oncol 2011; (Suppl): 4018
- Messager M, Lefevre JH, Pichot-Delahaye V, Souadka A, Piessen G, Mariette C. FREGAT working group - FRENCH. The impact of perioperative chemotherapy on survival in patients with gastric signet ring cell adenocarcinoma: a multicenter comparative study. Ann Surg 2011; 254 (05) 684-693 , discussion 693


 PDF
PDF  Views
Views  Share
Share

