A rare case of hepatoid carcinoma of the ovary with pancytopenia and hypocellular marrow
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2016; 37(04): 307-309
DOI: DOI: 10.4103/0971-5851.195744

Publication History
Article published online:
12 July 2021
© 2016. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Sir,
Hepatoid carcinoma of the ovary (HCO) is a rare extrahepatic α-fetoprotein (AFP) producing undifferentiated malignant tumor that has phenotypic properties in common with hepatocellular carcinoma (HCC). It has been primarily reported in postmenopausal women presenting with unilateral or bilateral ovarian masses.[1] Here, we describe a case of postmenopausal woman having HCO and pancytopenia. The bone marrow aspiration was hypocellular with infiltration of marrow with malignant cells.
A 47-year-old postmenopausal woman came to us because of complaints of fatigability, lethargy, and breathlessness on exertion for 1½ months. She also complained of pain abdomen of same duration. Ultrasonography of the abdomen done 15 days back showed right adnexal mass of 10 cm × 10 cm × 7 cm. Total abdominal hysterectomy and bilateral salpingo-oophorectomy were done in a periphery hospital for the same. At the time of admission, her vital parameters were stable. On examination, the patient was anemic, and there was no hepatosplenomegaly or lymphadenopathy. She denied any history of blood loss. Her hemogram showed pancytopenia with hemoglobin of 4.2 g/dL, leukocytes of 1500/mm3 with normal differential counts, and platelets of 62,000/mm3. The peripheral blood film examination showed normocytic normochromic red blood cells, leukopenia, and thrombocytopenia. A bone marrow aspiration done showed hypocellular marrow and infiltration of marrow with clumps of malignant cells [Figure [Figure1a1a and andb].b]. Histopathological examination of ovarian tissue showed characteristic features for hepatoid carcinoma. Hepatoid cells were polyhedral tumor cells with abundant eosinophilic cytoplasm and central round vesicular nuclei with variable prominent nucleoli in nests and trabeculae with a sinusoidal arrangement of vascular channels resembling liver [Figures [Figures1c1c and andd].d]. Serum AFP was 451.20 ng/mL (normal < 20 ng/ml) and carcinoembryonic antigen (CEA-125) was 325.60 unit/mL (normal – 35 unit/mL). Immunohistochemical staining of ovarian tissue was positive for AFP. Contrast-enhanced computerized tomography of the abdomen showed normal echotexture with normal size and shape of the liver. Mild peritoneal fluid was present in the peritoneal cavity. Ovaries were absent because of salpingo-oophorectomy. Gastroscopy was normal. She was tested negative for HIV, HBsAg, and hepatitis C virus. Chest X-ray, electrocardiogram, and echocardiogram were normal. Liver and renal function tests were within normal limit. She was treated for anemia by packed cell transfusion and expired 3 months after on chemotherapy.
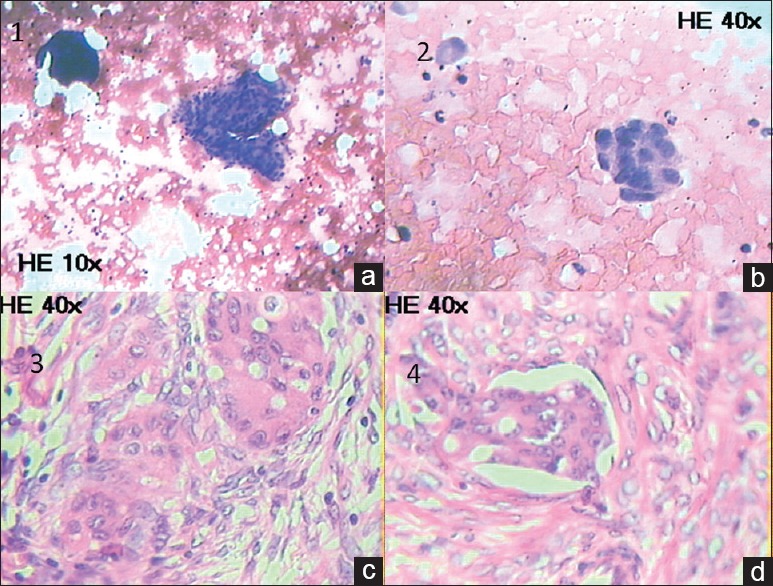
| Fig. 1 (a and b) Bone marrow aspiration showing hypocellular marrow and infiltration of marrow with clumps of malignant cells (a: H and E, ×10, b: H and E, ×40). (c) Histopathological examination of ovarian tissue showing hepatoid cells in a large group (H and E, ×40). (d) Ovarian section showing lymphovascular invasion of tumor (H and E, ×40)
Hepatoid carcinoma is less frequently occurring tumors and has mostly been found in the stomach but can also occur in other organs such as ovaries, lung, gallbladder, pancreas, and uterus. HCO is a rare ovarian invasive malignant tumor composed mainly of epithelioid cells. Histologically, the tumor resembled HCC by architectural and cytological features.[1,2] The diagnosis of HCO is based on the presence of abundant eosinophilic cytoplasm of the tumor cells that resemble HCC and positive immunohistochemical AFP staining.[3] HCO has been primarily reported in postmenopausal women presenting with unilateral or bilateral ovarian masses with elevated serum level of AFP. The common presenting symptoms are abdominal distension, abdominal pain, and pelvic mass.[4] The tumor may appear as entirely solid or with cystic areas, and there may be multiple foci of hemorrhage and necrosis. Serum AFP levels are universally high in HCO but less high as compared to serum cancer antigen-125. Hepatoid cells are similar to cells of HCC, characterized by solid sheets of large cells arranged, nests, or trabeculae, composed of cells with distinct borders with abundant eosinophilic cytoplasm, centrally located pleomorphic nuclei, and distinct cellular borders with usually absent bile canalicular structures.[4]
HCO is focally positive for AFP and polyclonal CEA (pCEA) on immunohistochemical staining. Among epithelial markers, cytokeratin-18 (CK) is focally positive while CK-19 and CK-20 are positive, suggesting an epithelial origin for HCO. These are negative in normal hepatocytes and HCC differentiating it from HCO. HCO can also be confirmed with diffusely positive staining pattern for CK-7 and hepatocyte paraffin-1.[4,5]
The differential diagnoses of HCO include hepatoid yolk sac tumor (HYST), endometrial carcinoma, clear-cell carcinoma, lipid cell tumor, and undifferentiated carcinoma.[3] HYST is similar to HCO in hepatoid differentiation and increased AFP production. In dissimilarity, HYST is considered as a germ cell tumor and occurs in a younger population (mean age 22 years) and is associated with gonadal dysgenesis and dysgerminoma.[4] The presence of surface epithelial markers supports a diagnosis of hepatoid carcinoma as opposed to HYST or other germ cell tumors. HYST stains negative for hepatocyte paraffin-1 and is focally positive for pCEA in contrast to HCO which is positive for hepatocyte paraffin-1 and with a diffuse membranous staining pattern for pCEA.
The mean age for presentation of HCO is 56 years.[4] Our patient presented at the age of 47 years with pancytopenia due to bone marrow infiltration with malignant cells, and recent surgery had been done for adnexal mass. The diagnosis of HCO was established on the basis of classical histopathological findings, immunohistochemistry, and marked elevation of AFP and CEA. Rarely, HCC may metastasize to the ovary, but the presence of classical immunohistochemistry and tumor markers favoring HCO in the presence of normal liver rules out this possibility.
In conclusion, HCO is a highly invasive malignant tumor in postmenopausal women. Involvement of the bone marrow in HCO is unusual and to the best of our knowledge, no case of HCO with metastatic infiltration of the bone marrow has been reported till date.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES

| Fig. 1 (a and b) Bone marrow aspiration showing hypocellular marrow and infiltration of marrow with clumps of malignant cells (a: H and E, ×10, b: H and E, ×40). (c) Histopathological examination of ovarian tissue showing hepatoid cells in a large group (H and E, ×40). (d) Ovarian section showing lymphovascular invasion of tumor (H and E, ×40)


 PDF
PDF  Views
Views  Share
Share

