A retrospective audit of clinicopathological attributes and treatment outcomes of adolescent and young adult non-Hodgkin lymphomas from a tertiary care center
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2011; 32(04): 197-203
DOI: DOI: 10.4103/0971-5851.95140
Abstract
Background: The uniqueness of adolescent and young adult (AYA) non-Hodgkin lymphomas (NHL) with respect to biology and treatment have largely remained unanswered due to marked heterogeneity in treatment, paucity of prospective, or retrospective studies and poor representation of AYA in clinical trials. This audit attempts to put forward the clinicopathological attributes and treatment outcomes of AYA NHL treated with both pediatric and adult protocols from a single centre in a developing country. Patients and Methods: Hospital records of all consecutive NHL patients registered in lymphoma clinic from January 2007 to May 2010 were reviewed for information on demography, clinical features, histology subtype, staging, treatment regimen, response rates, toxicities, and follow up. Two-year progression-free (PFS) and overall survival (OS) were calculated with Kaplan-Meier method. Results: AYA NHL constituted 4% of all lymphomas. Diffuse large B-cell (DLBL) was the most frequent subtype. Following were the 2-year PFS and OS - DLBL 64%, 76.9%, Burkitt′s lymphoma: 56%, 56%, lymphoblastic lymphoma: 33.2%, 44%. Our results did not show any improvement in outcome of DLBL with the use of Burkitt′s lymphoma like regimen. Conclusions: This study highlights some of the key features of AYA NHL occurring in developing world.
Keywords
Adolescent and young adults - adolescent and young adults malignancies - non-Hodgkin lymphomaPublication History
Article published online:
06 August 2021
© 2011. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:
The uniqueness of adolescent and young adult (AYA) non-Hodgkin lymphomas (NHL) with respect to biology and treatment have largely remained unanswered due to marked heterogeneity in treatment, paucity of prospective, or retrospective studies and poor representation of AYA in clinical trials. This audit attempts to put forward the clinicopathological attributes and treatment outcomes of AYA NHL treated with both pediatric and adult protocols from a single centre in a developing country.
Patients and Methods:
Hospital records of all consecutive NHL patients registered in lymphoma clinic from January 2007 to May 2010 were reviewed for information on demography, clinical features, histology subtype, staging, treatment regimen, response rates, toxicities, and follow up. Two-year progression-free (PFS) and overall survival (OS) were calculated with Kaplan-Meier method.
Results:
AYA NHL constituted 4% of all lymphomas. Diffuse large B-cell (DLBL) was the most frequent subtype. Following were the 2-year PFS and OS - DLBL 64%, 76.9%, Burkitt's lymphoma: 56%, 56%, lymphoblastic lymphoma: 33.2%, 44%. Our results did not show any improvement in outcome of DLBL with the use of Burkitt's lymphoma like regimen.
Conclusions:
This study highlights some of the key features of AYA NHL occurring in developing world.
INTRODUCTION
Issues pertaining to biology and therapy of malignancies occurring amongst adolescent and young adults (AYA) have largely remained neglected till last few years when data emerging from epidemiology and outcome registries suggested the need for active research in this field. Over the years, improvements in the understanding of biology and survival rates in this age group have lagged behind than those in pediatric and adult malignancies. The same story holds true for lymphomas which constitute 13% of all cancer diagnoses in AYA.[1,2] AYA non-Hodgkin lymphomas (NHL) have become a reason of concern among oncologists due to their rising incidence without a commensurate improvements in survival. The major reasons for the above observation are remarkable heterogeneity in the management of AYA NHL and lack of prospective trial for this subset of patients.[3] Diagnosis, staging, risk stratification and treatment protocols are largely dependent on whether the patient is referred to a pediatric or adult medical oncologist. These diversities in management make it difficult to compare the results from different protocols and identify the optimal therapy for this subgroup. Several retrospective analyses have suggested that pediatric protocol based treatment improves the event free and overall survival of AYA acute lymphoblastic leukemia. However, similar data is lacking for AYA NHLs. Considering AYA patients as older children and treating them with pediatric protocols might not be helpful in improving outcomes due to differences in toxicity patterns, drug pharmacokinetics, and disease biology. To compound this further, AYA NHLs neither features prominently in the clinical trials done in pediatric or adult NHLs nor any specific trials have been done for this age group.
These pertinent issues can only be resolved by the retrospective analysis of existing data from various centers and then designing appropriate prospective studies. Unfortunately, due to lack of data, most of the studies in oncology fail to incorporate the perspective of developing world in terms of disease nature and treatment outcomes.
The present retrospective analysis provides information on clinical presentation, histology patterns, and outcome results of AYA NHL treated with both adult and pediatric protocols at a tertiary cancer in India.
PATIENTS AND METHODS
Patients
Records of all consecutive patients with confirmed diagnosis of de-novo non-Hodgkin lymphoma (NHL) registered in lymphoma joint clinic at our hospital from January 2007 to July 2010 were reviewed in this audit. For this audit, we considered any patient between 15-25 years of age as AYA. Electronic medical records and hospital records were retrospectively reviewed and demographic details, duration of symptoms, presence or absence of B symptoms at diagnosis, clinical features, treatment regimen, and follow up information were collected. Evaluated parameters and initial staging procedures included physical examination, performance status as per Eastern Cooperative Oncology Group (ECOG) scale, complete blood count with differential, serum chemistry panel, LDH levels, chest radiography, CT scan of the thorax, abdomen and pelvis or PET-CT and bone marrow biopsy. Patients were staged according to the Ann Arbor system. Patients were assigned to one of four prognostic scores according to the International Prognostic Index (IPI) that takes into account the presence or absence of prognostic factors (serum LDH level, age, stage, performance status, and number of extranodal sites).
Pathology
Histopathological diagnosis in all cases was reviewed and reported by the expert hematopathologists as per WHO 2008 classification for hematolymphoid neoplasms.[4] Only patients with aggressive NHL like Burkitt's lymphoma (BL), lymphomas intermediated between Burkitt's and Diffuse large B-cell (Burkitt's-like), diffuse large B-cell lymphoma (DLBL), T-cell rich B-cell lymphoma, lymphoblastic lymphoma (LL), anaplastic large-cell lymphoma (ALCL) (alk-positive and alk-negative) and other aggressive T-cell lymphomas were included in the analysis.
Treatment, response assessment, and follow up
Treatment was done according to the lymphoma joint clinic decision. Various chemotherapy protocol used were- CHOP with or without rituximab, BFM-90, MCP-842, and MCP-841. MCP-842 protocol[5] is used at our centre for treatment of pediatric large-cell lymphomas and Burkitt's lymphomas. It is a short course, intensive, alternating cycle multiagent chemotherapy regimen based on cyclophosphamide, cytarabine, adriamycin, vincristine, etoposide, ifosfamide, and methotrexate. MCP-841 regimen was used for the treatment of acute lymphoblastic leukemia and lymphoblastic lymphomas.[6] Information regarding chemotherapy type and number of cycles received was recorded.
Response assessment was done as per the standards at our centre and it included clinical and radiological evaluation. Responses were classified into complete response, partial response, stable or progressive disease. Bone marrow biopsies were done at the end of therapy if it was involved at the baseline.
Incidence and severity of toxicities were recorded in form of number of episodes of febrile neutropenia, duration of hospitalization for management of febrile neutropenia, use of antifungals, use of inotropes, and intensive care support and organ toxicities.
Follow-up was done with clinical evaluation every 3 months for first 2 years and then every 6 months for the following 2 years. Relapses or progression were confirmed with radiological imaging and appropriate biopsies. Dates of relapse, progression, death and last follow up were recorded. Disease status at last follow up was noted down from the records.
Statistical analysis
Descriptive statistical analysis was done for demographic variables, clinical features, serum LDH levels, serum albumin, stage, IPI score, histopathologic subtype, type of chemotherapy received, response rates, relapse, progression, and toxicities.
Overall survival was calculated from date of registration to the date of death due to disease or any other cause or last date of follow up. Progression-free survival was calculated from the date of registration to the time of relapse, disease progression, death due to disease or treatment related complications or last follow up. Kaplan–Meier curves were generated for the overall and progression-free survival. SPSS version 16 was used for all analyses.
RESULTS
A total of 2745 patients with confirmed diagnosis of NHL were registered in lymphoma joint clinic at our centre from January 2007 to July 2010. One hundred and fourteen patients were between 15–25 years of age and had aggressive NHL. Complete information regarding treatment and response was available in 100 AYA patients. The demography, clinical features, distribution of histopathologic subtypes, stage at diagnosis and IPI scores in the group with complete information were similar to the whole group of 114 patients. The remaining patients did not complete staging work up (ten patients) or were not treated at our centre subsequently (four patients). The median age at diagnosis was 20.5 years. Male to female ratio was 4.9:1. A significant proportion of patients had B symptoms (53%) and bulky disease (58%) at presentation. Approximately two-third of patients had good performance status at presentation. Sixty percent of patients had stage III/IV disease at presentation. Extranodal disease involving bones, gastrointestinal tract, paranasal sinuses and ovaries was seen in 43% of patients. Seventy-eight percent patients had raised serum LDH levels. Low serum albumin (<3 href="https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3343245/table/T1/" target="table" class="fig-table-link figpopup" rid-figpopup="T1" rid-ob="ob-T1" co-legend-rid="" xss=removed>Table 1].
Table 1
Baseline characteristics of AYA NHL
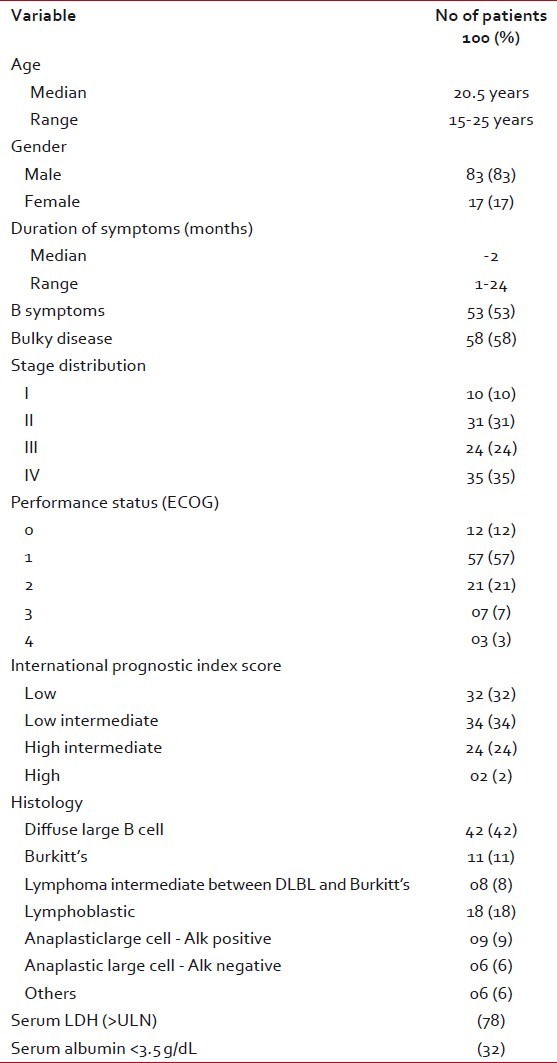
| Distribution of histopathologic subtypes in order of their frequency was as follows. Diffuse large B-cell: 42%, T-lymphoblastic lymphomas: 18%, Burkitt's lymphoma: 11%, anaplastic large-cell lymphomas-alk-positive: 9%, lymphomas intermediate between DLBL and Burkitt's: 8%, anaplastic large-cell lymphoma-alk- negative: 6%, others (mantle cell lymphoma, peripheral T-cell lymphoma- NOS, T/NK cell lymphoma, hepatosplenic gamma- delta T-cell lymphoma) 6%.
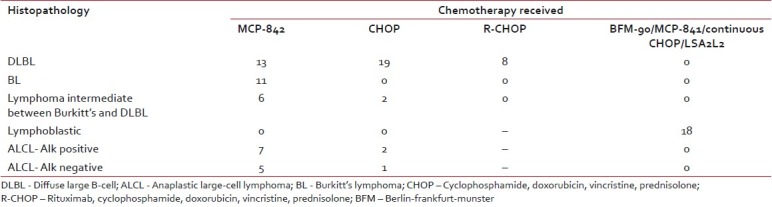
Description of chemotherapy protocols as per histopathological subtypes is given in Table 2. Patients with DLBL (40 patients) and lymphoma intermediate between DLBL and Burkitt's were treated with CHOP, R-CHOP or MCP-842 protocol as per the decision in lymphoma clinic. Two patients in DLBL category had primary CNS lymphoma and received treatment as per De-Angelis regimen. All patients with Burkitt's lymphoma received treatment with MCP-842 regimen. Patients with ALCL were treated predominantly with MCP-842 regimen. Chemotherapy regimens used for lymphoblastic lymphoma were very variable (BFM-90, MCP-841, Continuous CHOP, and LSA2L2) and were decided based on the socioeconomic status of the patients.
Table 2
Distribution of chemotherapy regimen among various histopathological subtypes
| Complete response rates at the completion of treatment amongst different histological subtypes (irrespective of the chemotherapy used) were DLBL: 67.5%, BL: 63%, lymphoma intermediate between DLBL and Burkitt's: 100%, ALCL-alk positive: 100%, ALCL- alk negative: 100%, LL- 44% [Table 3]. Two patients with primary CNS DLBL were in complete remission at the end of treatment.
Table 3
Responses to various chemotherapeutic regimens in individual histopathological subtype
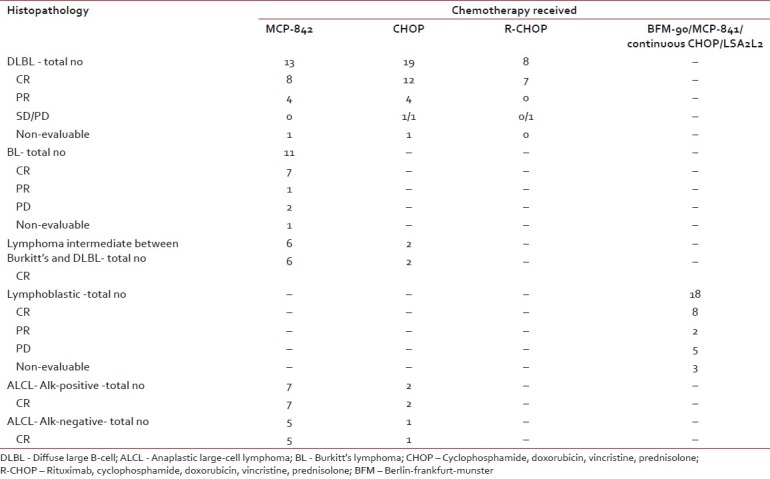
| Among patients with systemic DLBL complete remission was seen in 61.5%, 63%, and 87.5% patients treated with MCP-842, CHOP, and R-CHOP regimen, respectively. One patient each in MCP-842 regimen and CHOP was on therapy at the time of this analysis thus nonevaluable for the response.
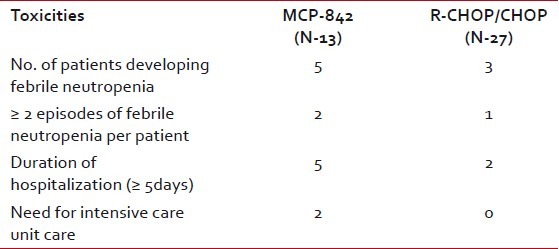
Therapy was well tolerated in majority of patients. Forty-five percent of patients developed febrile neutropenia requiring hospitalization. Twenty-five percent of patients developed ≥2 episodes of febrile neutropenia. Median duration of hospitalization was 3 days (range 0-52 days). Among patients requiring hospitalization 45% remained in-patients for ≥5 days. Requirement of inotropic support, care at intensive care unit, and antifungal therapy was seen in 2%, 4%, and 7%, respectively. There were two treatment related deaths. Table 4 describes the details of toxicity differences among different chemotherapeutic regimen in DLBL patients. There was one treatment related death in MCP-842 regimen due to bleeding secondary to grade-4 thrombocytopenia.
Table 4
Details of toxicities in patients with diffuse large B-cell lymphoma treated with different chemotherapy regimen
| The 2-year progression-free survival (PFS) for various histopathologic subtypes was as follows DLBL: 64%, BL: 56%, lymphoma intermediate between DLBL and Burkitt's: 70%, ALCL-alk positive: 85.7%, ALCL-alk negative: 66.7%, and LL: 33.2% [Figure 1].
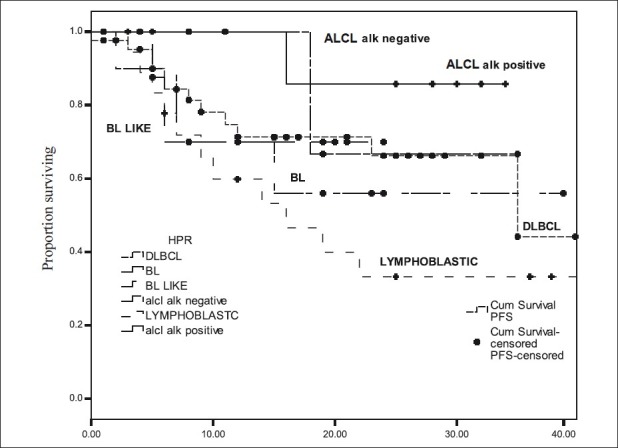
| Figure 1:Progression free survival for different subtypes of non-Hodgkin lymphoma
Two-year overall survival (OS) rates were DLBL: 76.9%, Burkitt's lymphoma: 56%, lymphoma intermediate between DLBL and Burkitt's: 80%, ALCL- alk positive and alk-negative: 100%, and LL: 44% [Figure 2].
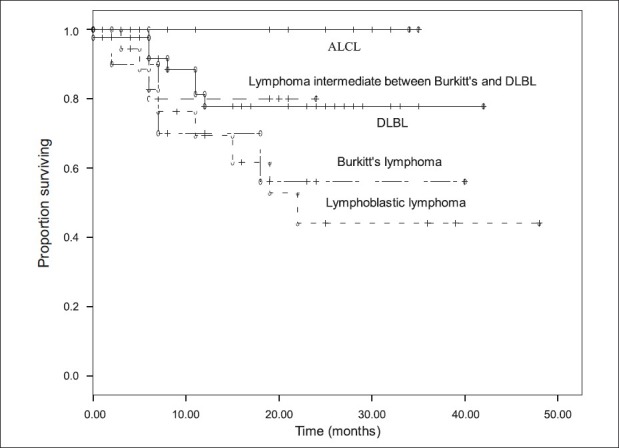
| Figure 2:Overall survival for different subtypes of non-Hodgkin lymphoma
Among 42 patients of DLBL subgroup the 2-year PFS and OS according to different chemotherapy regimens were following. MCP-842 51% and 69.2%, CHOP: 65% and 80%, R-CHOP: 83.3%, and 88.7% [Figures [Figures33 and and44].
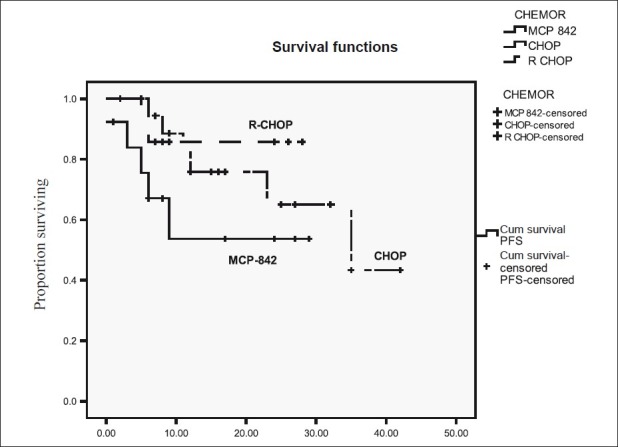
| Figure 3:Progression free survival for diffuse large B-cell lymphoma according to type of chemotherapy
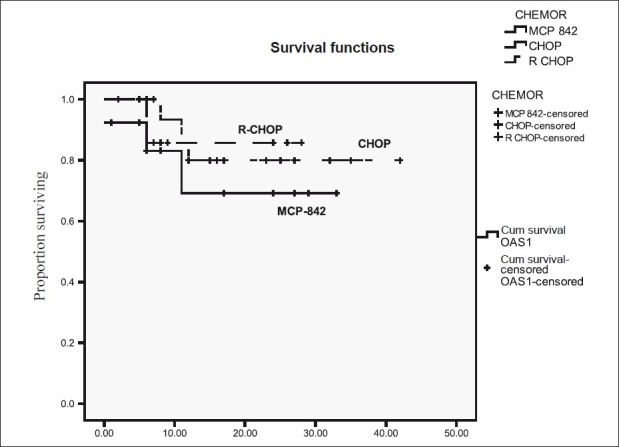
| Figure 4:Overall survival for diffuse large B-cell lymphoma according to type of chemotherapy
DISCUSSION
This single centre retrospective analysis highlights few relevant features of AYA NHLs in our country. In this study, AYA aggressive NHL constituted 4% of all lymphoma cases registered during the study period. Increased propensity of males to develop NHL as reported in SEER data could not reasonably explain the striking disproportionate male to female ratio of 4.9:1. Gender bias in seeking medical care is a more likely reason as seen for other diseases in our country. Clinical features associated with adverse outcomes like B symptoms, bulky disease, raised LDH, and advanced stage at presentation figured prominently in our patient population. This observation differs from literature where in more than 50% of adult patients are reported to have stage I or II disease at diagnosis. Delay in seeking medical care cannot be the sole reason for the high frequency of poor prognostic clinical correlates as the median duration of symptoms were only 2 months. It would be pertinent to study the underlying biology to answer these clinical differences.
The distribution of histological subtypes has few similarities and differences in comparison to the reported literature.[7] DLBL was the most frequent subtype in our study followed by lymphoblastic lymphomas-T cell. There is a conspicuous lack of data on frequency distribution of Lymphomas intermediate between Burkitt's and DLBL and alk-negative anaplastic large cell lymphomas in literature that constituted approximately 9% of AYA NHL in our analysis.
The choice of therapy for DLBL in AYA population remains difficult and controversial. Based on the referral, these patients are treated with chemotherapy regimens used in either adults or pediatric patients. Treatment of adult patients with CHOP based regimen has resulted in 5-year OS of only 30-40%.[8] Subsequent studies demonstrated that addition of rituximab lead to significant improvement in 2-year event-free survival (EFS) to 57% from 38% and 2 year OS to 70% from 57%. In contrast to adults, children with DLBL are treated with Burkitt's lymphoma like regimen with 5-year event-free survival of 89–97%.[9] The impressive survival rates in pediatric DLBL population with Burkitt's lymphoma like regimen makes the oncologist feel that AYA NHL should be treated with intensive regimens as they are older children. However, due to lack of retrospective or prospective comparisons it remains unclear whether increase toxicities and toxic deaths on intensive regimens can offset the benefit. Our analysis failed to show any remarkable difference in outcomes with the use of Burkitt's lymphoma like regimen in patients DLBL. Potential explanations for this lack of benefit are smaller number of patients, inclination to use intensive regimens in patients with poor risk disease, higher toxicity leading to treatment delays. The best results in our patients were obtained with R-CHOP (2-year PFS: 83.3%, 2 year OS: 85.7%); however, the number of patients in this subgroup were few.
There has not been much literature on the incidence and optimal management of lymphomas intermediate between DLBL and Burkitt's as it has come in to existence after WHO 2008 classification. The limited information available on its therapy suggests the use of Burkitt's lymphoma like regimens. At our centre, we have treated these patients with both MCP-842 regimen and CHOP chemotherapy with 2-year PFS and OS of 70% and 80%, respectively.
The treatment of Burkitt's lymphomas has seen great advances since its first recognition. Currently practiced regimen in pediatric population like LMB- 96 and BFM-90 protocols achieve 4-year EFS of 92%.[9] Two-year EFS of 92% have been obtained in adult patients too with protocols like CODOX-M/IVAC and Hyper-CVAD.[10–12] In this analysis, patients with Burkitt's lymphoma have shown relatively inferior results (complete response rate of 63% with 2-year PFS of 56%) as compared to the results reported in the literature for pediatric and adult population (complete remission rates of 97% and EFS of 92% at 2 years). This may be due to poor tolerance of therapy, poor risk features like advanced stage at presentation and delay in starting therapy due to financial constraints.
The outcome of lymphoblastic lymphoma is inferior in this analysis with 2-year PFS and OS rates of 33.2% and 44%, respectively. The treatment outcomes with various regimens have been in excess of 70% for patients in age group of 15-50 years.[13,14] Use of multiple regimens, advanced stage of disease was the potential reasons for this inferior outcome.
Alk-negative ALCL is seen more frequently in older adults and Alk-positive ALCL is the predominantly the disease of children and young adults. In our study, the frequency of both subtypes of ALCL was similar.
In pediatric population strategy of intensive therapy is efficacious and it results in overall survival of 65% at 5 years for patients with Alk+ ALCL.[15–17]
Alk-negative ALCL has relatively poor outcome with 5 year OS of 49%.[18] Several attempts to improve its outcome in adult patients have met with failures. Most of the patients are treated with CHOP like regimens. In this retrospective analysis, patients with both alk-negative and positive ALCL received treatment with MCP-842 regimen predominantly. The 2-year PFS rates in alk-negative and alk-positive groups were 66.7% and 85.7%, respectively.
This study is the only study reporting the disease patterns and treatment outcome results for AYA NHL from the developing country. As for developed world, differences in biology and uniqueness of this disease will hold true in developing countries too. However, there are several other important issues relevant to the developing world which needs to be addressed for improving the treatment results. These include poor compliance, lack of financial help for treatment and higher toxicities due to poor nutritional status of the host.
CONCLUSIONS
This is a very small and retrospective study of AYA NHL and thus it has several limitations in terms of applicability of the results. However, it does give an insight on the various aspects of clinical presentation and treatment outcome in this subgroup.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer Statistics 2009. CA Cancer J Clin 2009;59:225-49.
- O′Leary M, Sheaffer J, Keller F, Shu XO, Cheson B. Lymphomas and reticuloendothelial neoplasms. In: Bleyer A, O′Leary M, Barr R, Ries LA, editors. Cancer epidemiology in older adolescents and young adults 15 to 29 years of age, including SEER incidence and survival: 1975-2000. NIH Pub. No. 06-5767. Bethesda, MD: National Cancer Institute; 2006.
- Bleyer A. The adolescent and young adult gap in cancer care and outcome. Curr Probl Pediatr Adolesc Health Care 2005;35:182-217.
- Harris NL, Swerdlow SH, Jaffe ES, et al.. WHO classification of tumors of hematopoietic and lymphoid tissues. Vol. 4. Lyon: International Agency for Research on Cancer: 2008.
- Advani S, Pai S, Adde M, Vaidya S, Vats T, Naresh K, et al. Preliminary report of an intensified, short duration chemotherapy protocol for the treatment of pediatric non-Hodgkin′s lymphoma in India. Ann Oncol 1997;8:893-7.
- Raje SN, Vaidya SJ, Kapoor G, Pai SK, Nair CN, Kurkure PA, et al. Low incidence of CNS relapse with cranial radiotherapy and intrathecal methotrexate in acute lymphoblastic leukemia. Indian Pediatr 1996;33:556-60.
- Jaglowski SM, Linden E, Termuhlen AM, Flynn JM. Lymphoma in adolescents and young adults. Semin Oncol 2009;36:381-418.
- Fisher RI, Gaynor ER, Dahlberg S, Oken MM, Grogan TM, Mize EM, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin′s lymphoma. N Engl J Med 1993;328:1002-6
- Reiter A, Schrappe M, Tiemann M, Ludwig WD, Yakisan E, Zimmermann M, et al. Improved treatment results in childhood B-cell neoplasms with tailored intensification of therapy: A report of the Berlin-Frankfurt-Munster group trial NHL-BFM - 90. Blood 1999;94:3294-306.
- ;Moleti ML, Testi Am, Giona F, Malandruccolo L, Pescarmona E, Martino P, et al. CODOX-M/IVAC (NCI 89-C-41) in children and adolescents with Burkitts Leukemia/Lymphoma amd large B-cell lymphomas: A 15-year monocentric experience. Leuk Lymph 2007;48:551-9.
- ;Blum KA, Lozanski G, Byrd JC. Adult Burkitt leukemia and lymphoma. Blood 2004;104:3009-20.
- Thomas DA, Cortes J, O′Brien S, Pierce S, Faderl S, Albitar M, et al. Hyper-CVAD program in Burkitt′s-type adult acute lymphoblastic leukemia. J Clin Oncol 1999;17:2461-70.
- Reiter A, Schrappe M, Ludwig WD, Tiemann M, Parwaresch R, Zimmermann M, et al. Intensive ALL-type therapy without local radiotherapy provides a 90% event-free survival for children with T-Cell lymphoblastic lymphoma: A BFM group report. Blood 2000;95:416-21.
- Hoelzer D, Gokbuget N, Diget W, Faak T, Kneba M, Reutzel R, et al. Outcome of adult patients with T-lymphoblastic lymphoma treated according to protocols for acute lymphoblastic leukemia. Blood 2002;99:4379-85.
- Williams DM, Hobson R, Imeson J, Gerrard M, McCarthy K, Pinkerton CR; et al. Anaplastic large cell lymphoma in childhood: Analysis of 72 patients treated on the United Kingdom Children′s Cancer Study Group chemotherapy regimens. Br J Haematol 2002;117:812-20.
- Seidemann K, Tiemann M, Schrappe M, Yakisan E, Simonitsch I, Janka-Schaub G, et al. Short-pulse B-non-Hodgkins lymphoma-type chemotherapy is efficacious treatment for pediatric anaplastic large cell lymphoma: A report of the Berlin-Frankfurt-Munster group trial NHL-BFM-90. Blood 2001;97:3699-706.
- Lowe EJ, Sposto R, Perkins SL, Gross TG, Finlay J, Zwick D, et al. Intensive chemotherapy for systemic anaplastic large cell lymphoma in children and adolescents: Final results of Children′s Cancer Group study 5941. Pediatr Blood Cancer 2009;52:335-9.
- Fanin R, Sperotto A, Silvestri F, Cerno M, Geromin A, Stocchi R, et al. The therapy of primary adult systemic CD30-positive anaplastic large cell lymphoma: Results of 40 cases treated in a single center. Leuk Lymph 1999;35:159-69.

| Figure 1:Progression free survival for different subtypes of non-Hodgkin lymphoma

| Figure 2:Overall survival for different subtypes of non-Hodgkin lymphoma

| Figure 3:Progression free survival for diffuse large B-cell lymphoma according to type of chemotherapy

| Figure 4:Overall survival for diffuse large B-cell lymphoma according to type of chemotherapy
References
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer Statistics 2009. CA Cancer J Clin 2009;59:225-49.
- O′Leary M, Sheaffer J, Keller F, Shu XO, Cheson B. Lymphomas and reticuloendothelial neoplasms. In: Bleyer A, O′Leary M, Barr R, Ries LA, editors. Cancer epidemiology in older adolescents and young adults 15 to 29 years of age, including SEER incidence and survival: 1975-2000. NIH Pub. No. 06-5767. Bethesda, MD: National Cancer Institute; 2006.
- Bleyer A. The adolescent and young adult gap in cancer care and outcome. Curr Probl Pediatr Adolesc Health Care 2005;35:182-217.
- Harris NL, Swerdlow SH, Jaffe ES, et al.. WHO classification of tumors of hematopoietic and lymphoid tissues. Vol. 4. Lyon: International Agency for Research on Cancer: 2008.
- Advani S, Pai S, Adde M, Vaidya S, Vats T, Naresh K, et al. Preliminary report of an intensified, short duration chemotherapy protocol for the treatment of pediatric non-Hodgkin′s lymphoma in India. Ann Oncol 1997;8:893-7.
- Raje SN, Vaidya SJ, Kapoor G, Pai SK, Nair CN, Kurkure PA, et al. Low incidence of CNS relapse with cranial radiotherapy and intrathecal methotrexate in acute lymphoblastic leukemia. Indian Pediatr 1996;33:556-60.
- Jaglowski SM, Linden E, Termuhlen AM, Flynn JM. Lymphoma in adolescents and young adults. Semin Oncol 2009;36:381-418.
- Fisher RI, Gaynor ER, Dahlberg S, Oken MM, Grogan TM, Mize EM, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin′s lymphoma. N Engl J Med 1993;328:1002-6
- Reiter A, Schrappe M, Tiemann M, Ludwig WD, Yakisan E, Zimmermann M, et al. Improved treatment results in childhood B-cell neoplasms with tailored intensification of therapy: A report of the Berlin-Frankfurt-Munster group trial NHL-BFM - 90. Blood 1999;94:3294-306.
- ;Moleti ML, Testi Am, Giona F, Malandruccolo L, Pescarmona E, Martino P, et al. CODOX-M/IVAC (NCI 89-C-41) in children and adolescents with Burkitts Leukemia/Lymphoma amd large B-cell lymphomas: A 15-year monocentric experience. Leuk Lymph 2007;48:551-9.
- ;Blum KA, Lozanski G, Byrd JC. Adult Burkitt leukemia and lymphoma. Blood 2004;104:3009-20.
- Thomas DA, Cortes J, O′Brien S, Pierce S, Faderl S, Albitar M, et al. Hyper-CVAD program in Burkitt′s-type adult acute lymphoblastic leukemia. J Clin Oncol 1999;17:2461-70.
- Reiter A, Schrappe M, Ludwig WD, Tiemann M, Parwaresch R, Zimmermann M, et al. Intensive ALL-type therapy without local radiotherapy provides a 90% event-free survival for children with T-Cell lymphoblastic lymphoma: A BFM group report. Blood 2000;95:416-21.
- Hoelzer D, Gokbuget N, Diget W, Faak T, Kneba M, Reutzel R, et al. Outcome of adult patients with T-lymphoblastic lymphoma treated according to protocols for acute lymphoblastic leukemia. Blood 2002;99:4379-85.
- Williams DM, Hobson R, Imeson J, Gerrard M, McCarthy K, Pinkerton CR; et al. Anaplastic large cell lymphoma in childhood: Analysis of 72 patients treated on the United Kingdom Children′s Cancer Study Group chemotherapy regimens. Br J Haematol 2002;117:812-20.
- Seidemann K, Tiemann M, Schrappe M, Yakisan E, Simonitsch I, Janka-Schaub G, et al. Short-pulse B-non-Hodgkins lymphoma-type chemotherapy is efficacious treatment for pediatric anaplastic large cell lymphoma: A report of the Berlin-Frankfurt-Munster group trial NHL-BFM-90. Blood 2001;97:3699-706.
- Lowe EJ, Sposto R, Perkins SL, Gross TG, Finlay J, Zwick D, et al. Intensive chemotherapy for systemic anaplastic large cell lymphoma in children and adolescents: Final results of Children′s Cancer Group study 5941. Pediatr Blood Cancer 2009;52:335-9.
- Fanin R, Sperotto A, Silvestri F, Cerno M, Geromin A, Stocchi R, et al. The therapy of primary adult systemic CD30-positive anaplastic large cell lymphoma: Results of 40 cases treated in a single center. Leuk Lymph 1999;35:159-69.


 PDF
PDF  Views
Views  Share
Share

