A retrospective study of central venous catheters GCRI experience
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2013; 34(04): 238-241
DOI: DOI: 10.4103/0971-5851.125234
Abstract
Background: The use of central venous catheters (CVCs) has greatly improved the quality-of-care in cancer patients, yet these catheters may cause serious infectious and thrombotic complications. The aim of this retrospective study was to study the various types of CVCs and their complications. Materials and Methods: We studied retrospectively 213 cases of CVCs in our institute with their indications, type and complications from August 2010 to July 2011. Results: A total of 213 CVCs were inserted in patients with hematological (62%) and solid organ malignancies (38%). Ninety-eight patients (46%) had peripheral inserted central catheter (PICC), 90 (42%) patients had Hickman catheters and 25 (12%) had a port. The median duration of retention of Hickman catheters was 104 days (3-365 days), for the peripherally inserted central catheters was 59 days (3-100 days) and for the port it was 280 days (45-365 days). Non-infective complications were more than infective (12% vs. 7%). The most common complication was non-infective occlusion and thrombophlebitis. In one patient with PICC thrombosis occurred in the cephalic, radial and ulnar vein and in one patient with port thrombosis occurred in the superior vena cava. Organisms were isolated in 60% (12 out of 20) of cultures. Common organisms isolated were Pseudomonas aeruginosa in 5 (42%), Staphylococcus aureus in 2 (16%), Escherichia coli in 2 (16%) and Aspergillus in 3 (25%) patients. 7 out of 12 infected patients had negative blood cultures within 7 days of antibiotic treatment, 5 patients remained positive for more than 7 days with antibiotics. In 155 patients (73%), the desired treatment protocol was completed and at present there are still 28 patients (13%) with catheters. 5 patients (2.3%) died of febrile neutropenia and septicemia with multi-organ failure. In 5 patients (2.3%), the catheters (1 Port, 1 Hickman and 3 PICC) were prematurely removed because of thrombosis. Conclusion: CVCs are better options to facilitate the long-term vascular access provided infection is prevented with meticulous care and treated promptly with proper antibiotics. Most CVCs is acceptable to patients.
Keywords
Central venous catheter - chemo port - Hickman central venous catheter - peripheral inserted central catheterPublication History
Article published online:
19 July 2021
© 2013. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:
The use of central venous catheters (CVCs) has greatly improved the quality-of-care in cancer patients, yet these catheters may cause serious infectious and thrombotic complications. The aim of this retrospective study was to study the various types of CVCs and their complications.
Materials and Methods:
We studied retrospectively 213 cases of CVCs in our institute with their indications, type and complications from August 2010 to July 2011.
Results:
A total of 213 CVCs were inserted in patients with hematological (62%) and solid organ malignancies (38%). Ninety-eight patients (46%) had peripheral inserted central catheter (PICC), 90 (42%) patients had Hickman catheters and 25 (12%) had a port. The median duration of retention of Hickman catheters was 104 days (3-365 days), for the peripherally inserted central catheters was 59 days (3-100 days) and for the port it was 280 days (45-365 days). Non-infective complications were more than infective (12% vs. 7%). The most common complication was non-infective occlusion and thrombophlebitis. In one patient with PICC thrombosis occurred in the cephalic, radial and ulnar vein and in one patient with port thrombosis occurred in the superior vena cava. Organisms were isolated in 60% (12 out of 20) of cultures. Common organisms isolated were Pseudomonas aeruginosa in 5 (42%), Staphylococcus aureus in 2 (16%), Escherichia coli in 2 (16%) and Aspergillus in 3 (25%) patients. 7 out of 12 infected patients had negative blood cultures within 7 days of antibiotic treatment, 5 patients remained positive for more than 7 days with antibiotics. In 155 patients (73%), the desired treatment protocol was completed and at present there are still 28 patients (13%) with catheters. 5 patients (2.3%) died of febrile neutropenia and septicemia with multi-organ failure. In 5 patients (2.3%), the catheters (1 Port, 1 Hickman and 3 PICC) were prematurely removed because of thrombosis.
Conclusion:
CVCs are better options to facilitate the long-term vascular access provided infection is prevented with meticulous care and treated promptly with proper antibiotics. Most CVCs is acceptable to patients.
INTRODUCTION
Cancer patients require frequent intravenous administration of chemotherapy, antibiotics, blood components, etc., for a considerable period of time. Repeated venipunctures are poorly tolerated by these patients. The introduction of central venous catheters (CVCs) in the 1980s significantly improved the quality-of-life of oncology patients.[1,2] However; the use of these CVCs has been associated with mechanical, infectious and thrombotic complications.
Central venous devices are of various types like open-ended tunnelled catheters, tunnelled valve catheters and implanted subcutaneous ports, non-tunneled external catheters (CVCs and peripheral inserted central catheters (PICCs)).
Relative contraindications are bleeding disorders, anticoagulation or thrombolytic therapy, combative patients, distorted local anatomy, cellulitis, burns, severe dermatitis and vasculitis.
Selection of the device is based on the number of factors such as disease, number and type of solutions, osmolality, flow required and duration of use- days versus months, preference of physician and patient and cost of the device.
Complications are acute or delayed. Acute are: (1) procedure related: Dysrhythmias, catheter knotting or malposition, nerve injury, pneumothorax, hemothorax, hydrothorax, hemomediastinum; (2) vascular: Air embolus, arterial puncture, arteriovenous fistula, hematoma, blood clot; (3) infectious: sepsis, cellulitis, osteomyelitis, septic arthritis. Delayed like postinsertion phlebitis, extrinsic compression, i.e., pinch off, kink, catheter occlusion by precipitate, thrombus, fragmentation and infection.
AIMS AND OBJECTIVES
Aims
To study the profile of patients with CVCs and types of CVCs with respect to their complications.
Objectives
- Indications for various types of CVCs.
- Complications and their management overview.
MATERIALS AND METHODS
A retrospective study of function and complication rates of CVCs (n = 213) placed in children (112) and adults (101) at our institute over a 1 year period (August 2010 till July 2011) was done. We retrieved case files from the medical record department and reviewed for demographic profile, indication of insertion, any immediate, acute and late-onset complications. Indications of removal of catheter such as infection, occlusion and completion of treatment were noted.
Study population
Patients who were admitted to medical and pediatric oncology department underwent catheterization for various purposes were studied. The CVCs used were peripherally PICC, Hickman CVC (HC) or a chemo port (CP). A total of 458 CVCs were inserted. Patients with short-term catheter cavafix and subclavian/internal jugular vein (IJV) certofix (145) were excluded from the study. Patient with incomplete data (100) regarding CVC removal, lost to follow-up and catheter insertion outside the Gujarat Cancer Research Institute (GCRI) were also excluded from the study.
RESULTS AND ANALYSIS
In our study, median age of pediatric patients was 4 year (6 month to 14 year) and for adults 40 years (>14-65 year). The 112 (52.6%) CVCs were inserted in pediatric patients and 101 (47.4%) were in adult patients. Hickman was preferred in pediatric group, out of 112 CVCs, 68 (61%) were Hickman, 32 (28%) were PICC and 12 (11%) were port. Although in adults PICC was commonly used. Out of 101 CVCs, 66 (65%) were PICC, 22 (22%) were Hickman and 13 (13%) were port [Figures [Figures1,1, ,2,2, and Table 1].
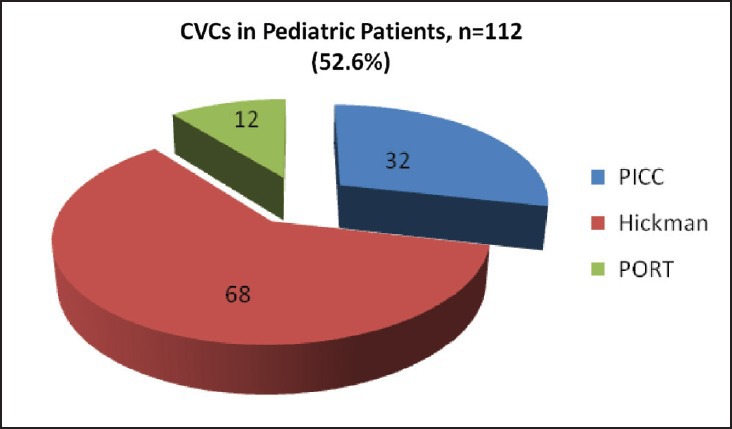
| Fig. 1 Number of pediatric patients with different central venous catheters
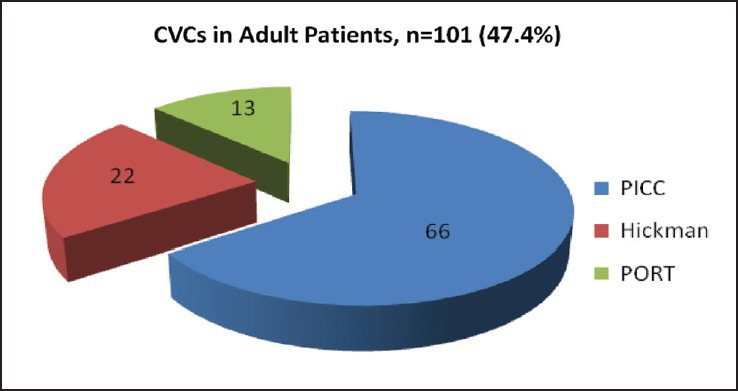
| Fig. 2 Number of adult patients with different central venous catheters
Table 1
Patient data

Overall 62% CVCs were used in hematological malignancies and 38% were used in solid malignancies. In pediatric patients, acute lymphoblastic leukemia was most common indication others were Ewing sarcoma, rhabdomyosarcoma, retinoblastoma, hepatoblastoma, neuroblastoma, wilms’ tumor and germ cell tumor. In adults most common indication was acute myeloblastic leukemia, others were breast cancer, colorectal cancer, head and neck cancer and non-Hodgkin's lymphoma.
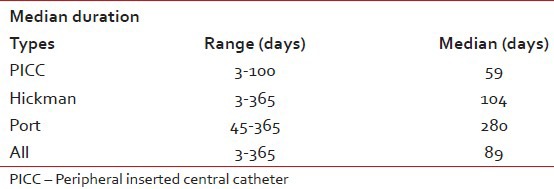
Overall median duration of CVCs was 89 days, for PICC 59 days, Hickman catheter 104 days and for port it was 280 days.
Catheter related complications were seen in 40 (19%) CVCs. Non-infective complications (12%) such as thrombophlebitis, malposition, swelling and occlusion were more common than infective (7%).
Table 2
Median duration of different devices
Blood cultures and/or catheter tip cultures were send in 20 cases. Organisms were cultured in 12 (60%) specimens. Most common organism was Pseudomonas (five cases) while Aspergillus (3), Staphylococcus aureus (2) and Escherichia coli (2) were found in others. 7 out of 12 infected patients had negative blood cultures within 7 days of antibiotic treatment while 5 patients remained positive. In 155 patients (73%), the desired treatment protocol was completed and at present there are still 28 patients (13%) with catheters. 5 patients (2.3%) died of febrile neutropenia and septicemia with multi-organ failure. In 5 patients (2.3%), the catheters (1 Port, 1 Hickman and 3 PICC) were prematurely removed because of thrombosis.
DISCUSSION
CVCs have a paramount role throughout the management of cancer patients, as they are needed in the initial phases for surgery or chemotherapy, in the advanced stage for chronic treatment and in the last stage for palliative measures. Central venous access is commonly attempted in the IJV, subclavian vein, femoral vein or arm veins.[3]
Physicians must determine the individualized catheter type by considering various factors such as catheter duration, technique, compliance, complications, cost and efficacy. CVCs are classified by tip position, technical features or materials. They can be classified in terms of short-term, medium-term or long-term access. Because superior vena cavas (SVCs) are a non-tunneled catheter, the expected duration was generally short and they have a known disadvantage of infection. The PICC is also a non-tunneled catheter and is useful for a relatively longer duration. The CP is a tunneled catheter and is useful for the long term. Tunneled catheters and PICCs[4,5] and are held to have lower infection rates, but no randomized controlled trials have demonstrated this contention to date.[6] Additionally, thrombosis occurs more often with a PICCs than other catheters due to the influence of multifactorial phenomenon.[7]
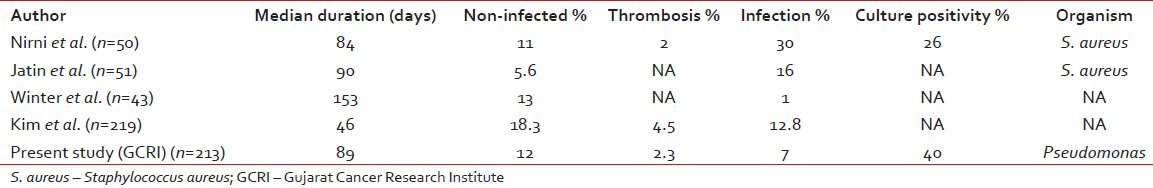
The incidence of catheter related complication was 19% in our study with 12% non-infected such as occlusion, malposition and swelling while 7% infected. In the study by Kim,[8] they had 18.3% non-infected complication like malposition, thrombosis, bleeding and 12.8% infected complications. In a prospective study by Nirni,[9] infection was found in 30% of cases with 26% culture positive, mainly for S. aureus. Jatin et al.[10] found infection in 16% cases. In the study by Winter et al.[11] 13% had a non-infective complication while only 1% had infective complication. The incidence of documented thrombosis was 2.3% in our study, which was relatively low despite not using prophylactic anticoagulation. One patient with acute myeloid leukemia (AML) developed thrombosis in the cephalic, radial and ulnar vein (PICC). Other two patients with PICC thrombosis were AML induction (1) and acute lymphocytic leukemia (ALL) consolidation (1). While one patient with ca breast on adjuvant chemotherapy developed thrombosis in SVC (Port) and another patient with ALL consolidation developed thrombosis in HC. The thrombosis incidence rate tended to be more frequent (three cases) in patients with PICCs than Hickman and CPs. Nirni and Kim et al. noticed 2% and 4.5% incidence of thrombosis in their studies respectively. In our study incidence of complications are less, probably secondary to proper counselling, strict aseptic precautions and proper cath flush. Furthermore, we have separate CVC care service and dedicated nursing staff.
Table 3
Comparison of different studies
We found one catheter-related immediate-onset complication in AML induction patient in the form of hemothorax after IJV insertion, that patient also had PICC related thrombosis and pulmonary embolism and was expired. No other immediate complication like pneumothorax, except bleeding was found. Most cases were performed by an expert anesthetist or by expert oncology resident. Majority of PICC insertion were without using ultrasound (USG) or fluoroscopic guidance. A study has also reported that USG is not beneficial for reducing catheter-related complication.[12] However, it is optimal to insert catheters using USG or fluoroscopic guidance if facility available to reduce the immediate-onset complication rate.
The median PICC life span was 59 days (3-100 days), for Hickman 104 days (3-365 days) and for port 280 days (45-365 days). Overall median duration was 89 days until study completed. In addition, a longer-term use of catheters occurred in patients with a solid malignancy than hematologic malignancy. Patients with a solid malignancy underwent a CP rather than another catheter type due to scheduled, intermittent long-term chemotherapy. Therefore, the CP was considered an effective tool for long-term use in patients with cancer. In Kim study, the median catheter life span was 46 days and the CP was useful for the long term (median 269 days); however, the median life span of the PICC was 37 days. In studies by Nirni, Jatin et al. and Winter et al. had median duration of 84 days, 90 days and 153 days respectively.
CONCLUSION
CVCs are better options to facilitate the long term vascular access provided infection is prevented with meticulous care and treated promptly with proper antibiotics. Most CVCs are acceptable to patients.
The major problems related to CVCs were thrombosis, malposition or migration of the tip and infection. Port is an effective tool for long-term use in patients with cancer, while Hickman and PICC are feasible for few months. In addition, the insertion of CVCs with the image guidance is advisable and the fixation of the tip is important for the management of PICC.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Bharat J. Parikh (MD, Professor and Chief of Medical Oncology Unit 1, Gujarat Cancer and Research Institute, Ahmedabad), Dr. Asha N Anand (MD, professor Department of Medical and Pediatric Oncology, Gujarat Cancer and Research Institute, Ahmedabad), nursing staff and patients of Gujarat Cancer and Research Institute.
Footnotes
Source of Support: Department of Medical and Pediatric Oncology and Department of Microbiology, Gujarat Cancer and Research Institute, Ahmedabad.
Conflict of Interest: None declared.
REFERENCES

| Fig. 1 Number of pediatric patients with different central venous catheters

| Fig. 2 Number of adult patients with different central venous catheters


 PDF
PDF  Views
Views  Share
Share

