A Significant Breakthrough in the Incidence of Childhood Cancers and Evaluation of its Risk Factors in Southern Iran
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(02): 158-164
DOI: DOI: 10.4103/ijmpo.ijmpo_40_16
Abstract
Background and Objective: This study investigates epidemiologic and practical information about the incidence and risk factors of childhood cancer in a population of Southern Iranian children. Materials and Methods: A total number of 300 cancer patients along with 600 age- and gender-matched healthy control were interviewed by a trained physician regarding their demographic characteristics, and major family-associated risk factors, childhood malignancies. Results: The average annual percentage change for cancers in the studied population is calculated as 45%. Our study indicated that possible risk factors which could contribute to the development of childhood cancer are maternal oral contraceptive pill use during pregnancy, exposure to radiation during pregnancy, parental smoking, residence near high voltage electricity lines, exposure to pesticides and fertilizers, patient allergy, contact with domestic animals and father's educational degree. Furthermore, new ecological risk factors such as air pollution due to nonstandard petroleum or toxic inhalant particles, nonhealthy food consumption, and satellite jamming are other predisposing factors. Conclusion: Our study reported a higher average annual percentage change of childhood cancers in our area, compared to the existing literature. In conclusion, detection and prevention of the consistent and possible new environmental risk factors such as nonstandard petroleum or satellite jamming from all around the country should be taking into consideration.
Publication History
Article published online:
06 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background and Objective:
This study investigates epidemiologic and practical information about the incidence and risk factors of childhood cancer in a population of Southern Iranian children.
Materials and Methods:
A total number of 300 cancer patients along with 600 age- and gender-matched healthy control were interviewed by a trained physician regarding their demographic characteristics, and major family-associated risk factors, childhood malignancies.
Results:
The average annual percentage change for cancers in the studied population is calculated as 45%. Our study indicated that possible risk factors which could contribute to the development of childhood cancer are maternal oral contraceptive pill use during pregnancy, exposure to radiation during pregnancy, parental smoking, residence near high voltage electricity lines, exposure to pesticides and fertilizers, patient allergy, contact with domestic animals and father's educational degree. Furthermore, new ecological risk factors such as air pollution due to nonstandard petroleum or toxic inhalant particles, nonhealthy food consumption, and satellite jamming are other predisposing factors.
Conclusion:
Our study reported a higher average annual percentage change of childhood cancers in our area, compared to the existing literature. In conclusion, detection and prevention of the consistent and possible new environmental risk factors such as nonstandard petroleum or satellite jamming from all around the country should be taking into consideration.
Introduction
Cancer is generally expected to be seen in adults, and only a small proportion of cancer reports belong to children. The overall incidence of childhood cancer varies from 75 to 150 per million children per year in different parts of the world, and this number seems to be rising.[1] Despite all the improvements in treatment and diagnostic techniques in the field of oncology during the past decades, cancer remains the second main reason of death in children <15 href="https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5582553/#ref2" rid="ref2" class=" bibr popnode tag_hotlink tag_tooltip" id="__tag_635949714" role="button" aria-expanded="false" aria-haspopup="true" xss=removed>2] Apart from this large amount of mortality, the psychological and financial burden of childhood cancer on their parents and also the society are other concerns in this field.[3,4]
Knowing about risk factors leading to cancer in children helps health-care providers in having a better approach to this issue. Moreover, with access to such information, more effective preventive actions could be undertaken.[5]
Studies suggest that predisposing factors to childhood cancer are genetic factors, radiations, maternal diabetes and other autoimmune diseases, prenatal exposures to viral infections, pesticides, ionizing and nonionizing radiations, parental occupation exposures to petrochemicals, hydrocarbons, metals and pesticides, and medical exposures including prenatal X-rays, chemotherapy, and other medications.[6,7,8]
At present, there are very little reliable and accessible information about risk factors of childhood cancer, especially in Iranian pediatric population. In this context, this study aims to provide epidemiologic and practical information about risk factors of childhood cancer including predisposing genetic and environmental factors in a population of Southern Iranian children.
Materials and Methods
In this case–control study, within a 3-year interval (January 2011–December 2013), all children (0–18 years old) with a diagnosis of malignancy, whether primary or relapse cases, whom were admitted to the pediatric oncology hospital affiliated to the Shiraz University of Medical Sciences; our referral center for pediatric oncology patients in Southern Iran, were included. Those patients who were not available during the study, families of deceased patients and individuals unwilling to participate were excluded. In this time interval, a total number of 327 pediatric patients were recruited, in which 27 individuals were excluded, leaving 300 cases. A total number of 600 age pair- and gender pair-matched noncancer children whom had referred to the outpatient pediatric clinics affiliated to the Shiraz University of Medical Sciences for other reasons such as gastroenteritis, pneumonia, common cold, asthma, renal disease, and seizure disorder were selected as the control group. The study protocol was approved by the Ethical Committee of Shiraz University of Medical Sciences, and all of participants/parents, after receiving an explanation of the study purpose and design, were provided with a written informed consent.
Measurements and data collection
To obtain data from study subjects, data gathering form was designed with four subsequent parts. In the first part, demographic characteristics (age, gender, area of residence, and birthplace) of the study subjects were recorded. In the second part, family-associated risk factors throughout the patient's lifetime including the parents’ job and level of education, history of smoking, and family history of any kind of malignancies diagnosed at any age, parents’ drug abuse, number of siblings and major medical conditions in the family were evaluated. Other possible risk factors such as residence near high voltage power (about 100 m near lines), exposure to pesticides, solvents and hydrocarbons (>4 times/month), use of wood burners at home along with contact with domestic animals were recorded in the third part. Natal characteristics including birth weight, type and complications of delivery, feeding during the first 2 years of life, and underlying medical conditions were also recorded.
Prenatal risk factors including age of parents at time of pregnancy, history and type of infertility treatment, oral contraceptive pill (OCP) use in the first 2 months of pregnancy, history of radiation exposure, drug and alcohol use along with infections and medical diseases of mothers during pregnancy in addition to ultrasonographic abnormalities were recorded in the fourth part. All of the data gathering forms were filled by a trained physician in the setting of an interview with the patients/parents and the control individuals. Moreover, all of the participants’ medical records we reviewed.
Statistical analysis
The incidence of cancers was calculated as per million children (0–18 years old) in the Fars Province. Data regarding the overall population of children in our province was extracted from, database accessible through the National Organization for Civil Registration of Iran website (www.sabteahval.ir). The average annual percentage change was calculated, using geometric mean.
Data analysis was presented as mean ± standard deviation. At first, each variable was separately entered in a univariate logistic regression model; then, the variables which had a P < 0 xss=removed>P < 0>
Results
Demographics
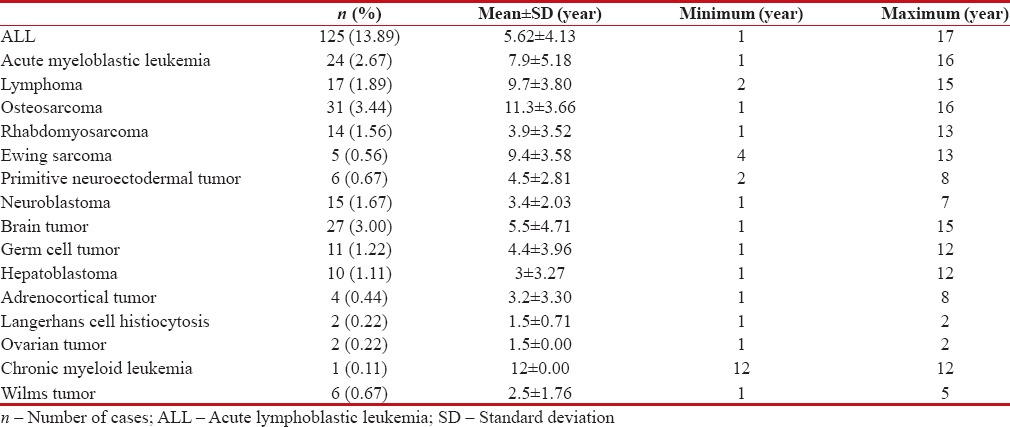
The mean age of participants in the case group was 6.21 ± 4.6 (range: 0–17) years while it was 6.3 ± 3.9 (range: 0–16) years in the control group. From male to female ratio was 1.2:1 in the case group and 0.9:1 in the control group, which was not statistically significant. Table 1 represents the age pattern of different childhood cancers, and Figure 1 illustrates the distribution of different cancers.
Table 1
Age pattern of different childhood cancers in Southern Iran
|
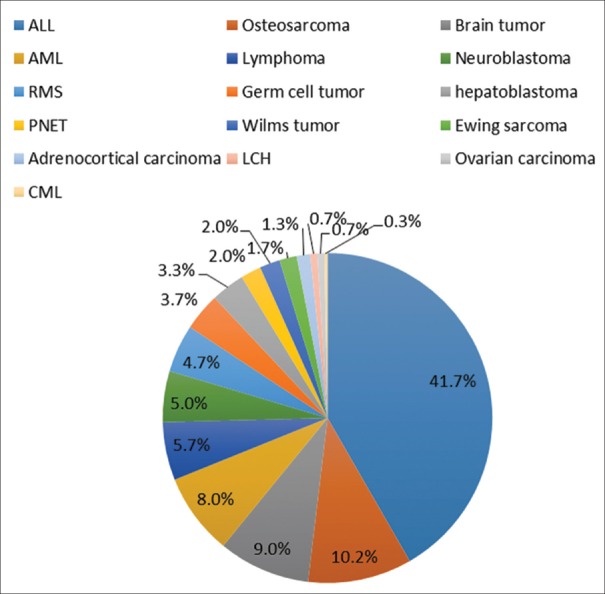
| Figure 1:Ratio of different malignancies in Southern Iran
Incidence of childhood cancer
With an overall population of 1,242,968 (ages 0–18 years) in the Fars Province, the incidence of malignancies in the studied population was 41 per million in the year 2011 while it was 68 per million in 2012 and 87 per million in 2013, with acute lymphoblastic leukemia (ALL) being at the top of the list. The average annual percentage change for cancers in children in the studied population is calculated as 45%. ALL incidence rate was 12 per million in 2011 followed by 28 per million in 2012 and 50 per million in 2013. Figure 2 shows the alterations in the incidence of childhood malignancies over the past 3 years.
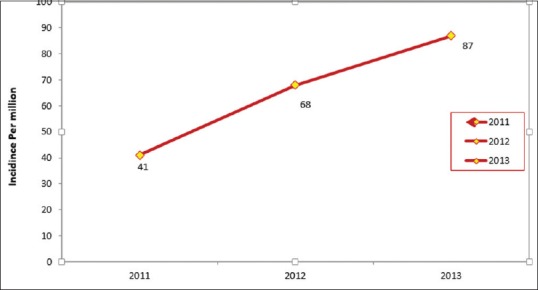
| Figure 2:Ratio oIncidence rate of cancers per million in children from 2011 to 2013
Based on the results of univariate logistic regression model analysis, characteristics such as age, gender, birthplace, area of residence, the parent's job, drug abuse in parents, order of birth, number of siblings, blood group, birth weight, prematurity, breastfeeding in the first 2 years of life, route of delivery, medications in pregnancy, abnormal prenatal ultrasonographic abnormalities, parent's age at time of pregnancy, and type of infertility treatment had P > 0.2 and so they were not included in the multiple logistic regression analysis.
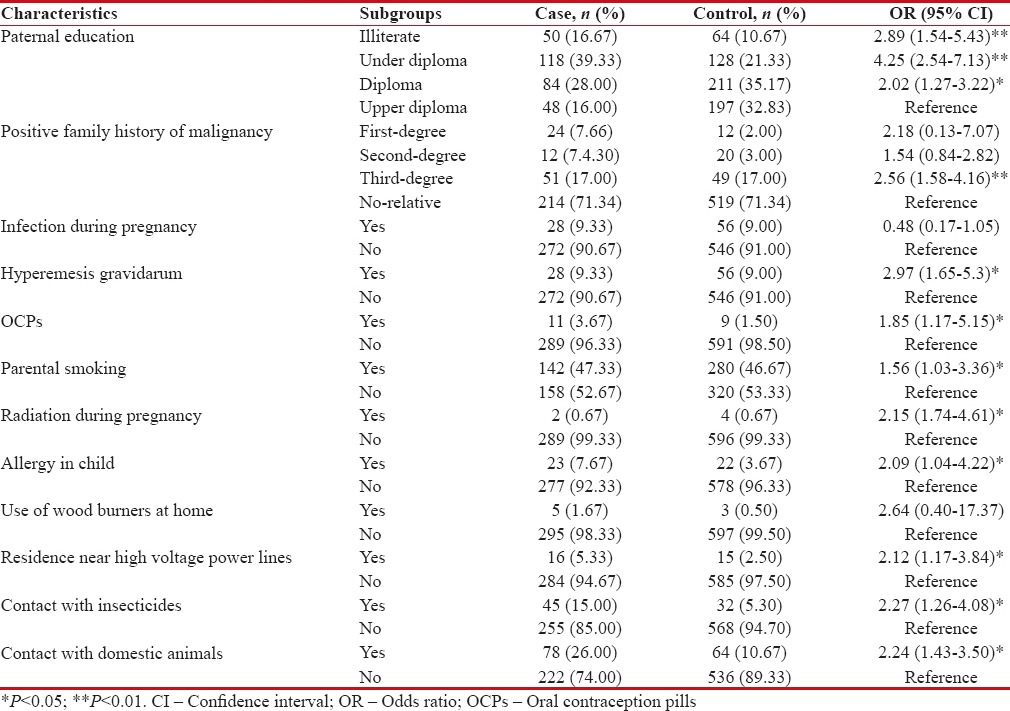
Table 2 demonstrates the results of the factors analyzed through multiple logistic regression model.
Table 2
Results of multiple logistic regression models for factors associated with malignancies in children
|
Prenatal- and natal-associated risk factors of childhood cancer
Eleven patients (3.7%) in the case group versus 9 (1.5%) patients in the control group had positive history of maternal OCP consumption in the first few months of pregnancy, which our analysis revealed a 1.85 times increase risk of cancer for the case versus control group (OR 1.85, 95% CI 0.67–5.15).
Logistic analysis revealed a 2.15 times increased risk of malignancy for those with exposure to radiation during pregnancy (OR 2.15, 95% CI 0.74–4.61). Furthermore, hyperemesis gravidarum during pregnancy had a 2.97 times increase risk malignancy in the child.
However, infections during pregnancy had no significant effect on malignancy.
There was a significant relationship between the patients’ allergy (mostly food allergy) and getting a malignancy (OR 2.09, 95% CI 1.04–4.22).
Family-associated risk factors and childhood cancer
The results showed that children who were exposed to parental smoking had 1.56 times greater risk of malignancy (OR 1.56, 95% CI 0.73–3.36).
Regarding the father's educational level, logistic regression showed children whom their fathers were illiterate, under diploma degree, and diploma degree, respectively, had 2.89, 4.25, and 2.02 times higher risk of cancer compared to children whom their fathers were with upper than diploma.
History of cancer among first- and second-degree relatives had no effect on cancer risk, but a history of cancer in third-degree relatives three-time increases risk of cancer (OR 2.56, 95% CI 1.58–4.16).
Environmental risk factors and childhood cancer
There was a statistically significant difference between the study groups regarding living near high voltage electricity lines (OR 2.12, 95% CI 1.17–3.84). Lymphoma (11.8%) followed by osteosarcoma (9.8%), neuroblastoma (6.7%), and ALL (6.4%) were the malignancies related to residence near high voltage electricity lines.
There was a 2.24 times increased the chance of acquiring a malignancy in children with a positive history of contact with domestic animals (mostly hens, roosters, and sheep) (OR 2.24, 95% CI 1.43–3.50). Malignancies related to patients’ contact with domestic animals was adrenocortical tumors (75%), hepatoblastoma (50%), brain tumors (37%), rhabdomyosarcoma (28.6%), and ALL (28%).
The difference between the case and control group was significant in terms of exposure to chemical pesticides and fertilizers (OR 2.27, 95% CI 1.26–4.08). Malignancies such as adrenocortical tumors (50%), hepatoblastoma (40%), brain tumor (22%), and Ewing's sarcoma (20%) were mostly related to chemical exposures.
Discussion
The present study was conducted as a case–control experiment with the aim of providing epidemiologic and practical information regarding risk factors of childhood malignancies including predisposing genetic and environmental factors in a population of Southern Iranian children. To the best of our knowledge, only one experiment with similar goals was conducted in Iran; therefore, the data presented here will have to be further confirmed by future studies.
As indicated in the section on the results, the overall incidence of malignancies in the studied population in Southern Iran has increased in the past 3 years. As the general lifestyle and trends of the Southern population of the country and the environmental risk factors, which we have also studied here, have not changed significantly over the past 3 years, the significant increase in the overall incidence of childhood malignancies could be attributed to probably some other new ecological risk factors such as air pollution due to nonstandard petroleum or toxic inhalant particles, nonhealthy food consumption and satellite jamming,[9,10,11] to which exposures are difficult to assess and actually out of our hands.
In this study, we have reported an average incidence of 65 per million per year of childhood cancers, with a mean annual increase rate of 45%. In a study by Siegel et al. which had analyzed data from the National Program of Cancer Registries and Surveillance in the United States reported an age-related incidence rate of 171.01 per million per year of childhood cancers in an 8-year time period, this study stated an increase in the overall cancer trend among African American children and adolescents with an annual percentage change of 1.3%.[12] Furthermore, in 2010, a data analysis on the Brazilian Population-Based Cancer Registry reported an age-adjusted cancer rate of 154.3 per million in children.[13] Articles from France and Mexico have also reported incidences as high as 156.6 versus 150.3 per million, respectively.[14,15] Another study conducted in India, in 2008, showed age-standardized rates for all childhood cancers of 127 per million for boys and 88 per million for girls and also stated a decreasing trend in incidence rates.[16] However, our results seem not to be comparable with the above results due to the fact that our data were collected from a limited area of the country (Southern Iran) which includes only 5% of the pediatric population of the republic, therefore, we cannot generalize these statics to the overall pediatric population of Iran. Yet this study has shown a considerable increase in the trends of childhood cancers from our area in the past 3 years.
The most prevalent malignancies in the studied population were, ALL (41.7% of cases) followed by osteosarcoma (10.3%) and brain tumors (9%). Our results are in agreement with results of two other studies performed in the Northern part of Iran.[17,18]
Overall, our study indicated that possible risk factors which could contribute to the development of childhood cancer are maternal OCP use during pregnancy, exposure to radiation during pregnancy, parental smoking, residence near high voltage electricity lines, exposure to pesticides and fertilizers, patient allergy, contact with domestic animals and father's educational degree.
Regarding family-associated risk factors, parental smoking was considered a risk factor for pediatric malignancies in our study. Our findings were in contrast to those of Edraki and Rambod, who conducted a case–control study in Shiraz-Southern Iran, aimed to determine the relationship between parental smoking and childhood cancer.[19] Still, epidemiological studies investigating family history of cancer have not provided conclusive evidence for associations of parental smoking with childhood malignancies.[20]
There was a significant difference between the study groups in terms of fathers’ educational degree, i.e. cancer patients comparing to controls had fathers with lower educational degrees. Although little has been reported on socioeconomic status patterns of risk for most forms of childhood cancers, results of two recent reviews have not documented a strong relationship between paternal educational attainments and risk of childhood cancers for most of malignancies.[21,22]
In our experiment, there was a significant difference between the case and control group regarding the unintentional consumption of oral contraceptives during the first trimester of pregnancy. Our results are in agreement with findings of other experiments.[23,24]
Results of the present experiment indicated that lymphoma (11.8%) followed by osteosarcoma (9.8%), neuroblastoma (6.7%), and ALL (6.4%) were the malignancies related to residence near high voltage electricity lines (about 100 m around lines). Epidemiologic studies have reported associations between measures of power-line electric or magnetic fields and childhood leukemia.[25,26]
Our research revealed a significant relationship between childhood cancers and exposure to chemical pesticides and fertilizers. Most previous epidemiologic studies have also examined the impact of paint and solvents on the risk of childhood malignancies through parental occupational exposures and have reported conflicting results.[27,28,29,30,31,32,33]
Our study indicated a relationship between exposure to domestic animals and the risk of cancer. Despite the fact that few studies exist in the literature addressing the association between childhood cancer and exposure to domestic animals, none have issued a strong relationship.[34,35]
We indicated a significant association of allergy (mostly food allergy) and childhood cancer, however, it should be mentioned that there are inconsistencies in literature regarding the association between patient allergy/atopy and risk of childhood malignancies.[36]
Possible limiting factors which could diminish the impact of this experiment were a relatively small number of studied individuals, methodological weaknesses in terms of design of the study, lack of organized specified exposure assessment procedures, recall bias regarding the parents, and heterogeneous group of studied malignancies along with limited number of studied individuals with rare malignancies. Meanwhile, it is important to note that the information about childhood cancer incidence in countries such as Iran comes from hospital-based registries, population-based registries, international organizations, and specific research projects. This heterogeneity of the sources of data affects the overall reported incidence of childhood malignancies and further diminishes the impact of studies coming from developing countries. The main advantages of the present experiment could be the extent of malignancies studied, evaluation of a wide variety of cancer risk factors in population, proper classification of studied risk factors and proving basic epidemiologic evidence regarding pediatric malignancies in Southern part of the country.
Conclusion
This epidemiologic study reported a higher average annual percentage change of childhood cancers in our area, compared to the existing literature. Yet the overall, findings of risk factors of childhood cancer are in agreement with the previous studies. Regarding the increase in the incidence of childhood cancer from our area, it is proposed that a national cancer registry program should be designed with the aim of detecting and preventing the consistent and also the possible hazardous new environmental risk factors from all around the country. Moreover, major health politics should be redirected in terms of providing rapid access to primary, secondary and tertiary medical care centers (a pediatric cancer unit), better recognition of cancer symptoms, correct diagnosis, and data management infrastructures.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This article was adopted from Maryam Niknam's thesis for Specialization in Pediatrics.
References
- Parkin DM, Stiller CA, Draper GJ, Bieber CA. The international incidence of childhood cancer. Int J Cancer 1988;42:511-20.
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917.
- Alderfer MA, Cnaan A, Annunziato RA, Kazak AE. Patterns of posttraumatic stress symptoms in parents of childhood cancer survivors. J Fam Psychol 2005;19:430-40.
- Luo W, Lane R, Stobart K, Morrison H, Schanzer D, Barr R, et al. The medical care cost of childhood and adolescent cancer in Manitoba, 1990-1995. Chronic Dis Can 2002;23:83-90.
- Asselin BL. Epidemiology of childhood and adolescent cancer. 19th ed. Philadelphia: Lippincott Williams & Wilkins; 2011. p. 1725-8.
- Savitz DA, Chen JH. Parental occupation and childhood cancer: Review of epidemiologic studies. Environ Health Perspect 1990;88:325-37.
- Westbom L, Aberg A, Källén B. Childhood malignancy and maternal diabetes or other auto-immune disease during pregnancy. Br J Cancer 2002;86:1078-80.
- Chow WH, Linet MS, Liff JM, Greenberg RS. Cancers in children. In: Schottenfeld D, Fraumeni JF Jr., editors. Cancer Epidemiology and Prevention. 2nd ed. New York: Oxford University Press; 1996. p. 1331-69.
- Namehnews.ir. Prevalence of Leukemia in Children. Available from: http://namehnews.ir/fa/news/171764.
- Fararunews. Toxic Small Particles Reach the Capital. Available from: http://www.fararu.com/fa/news/206747.
- ;IRNA. Parasites in Iran: Yes or No? News Code: (4201730) 81292960. Available from: http://www.irna.ir.
- Siegel DA, King J, Tai E, Buchanan N, Ajani UA, Li J. Cancer incidence rates and trends among children and adolescents in the United States, 2001-2009. Pediatrics 2014;134:e945-55.
- de Camargo B, de Oliveira Santos M, Rebelo MS, de Souza Reis R, Ferman S, Noronha CP, et al. Cancer incidence among children and adolescents in Brazil:First report of 14 population-based cancer registries. Int J Cancer 2010;126:715-20.
- Lacour B, Guyot-Goubin A, Guissou S, Bellec S, Désandes E, Clavel J. Incidence of childhood cancer in France: National Children Cancer Registries, 2000-2004. Eur J Cancer Prev 2010;19:173-81.
- Rivera-Luna R, Correa-González C, Altamirano-Alvarez E, Sánchez-Zubieta F, Cárdenas-Cardós R, Escamilla-Asian G, et al. Incidence of childhood cancer among Mexican children registered under a public medical insurance program. Int J Cancer 2013;132:1646-50.
- Swaminathan R, Rama R, Shanta V. Childhood cancers in Chennai, India, 1990-2001: Incidence and survival. Int J Cancer 2008;122:2607-11.
- Moradi A, Semnani S, Roshandel G, Mirbehbehani N, Keshtkar A, Aarabi M, et al. Incidence of childhood cancers in Golestan Province of Iran. Iran J Pediatr 2010;20:335-42.
- Mousavi SM, Pourfeizi A, Dastgiri S. Childhood cancer in Iran. J Pediatr Hematol Oncol 2010;32:376-82.
- ;Edraki M, Rambod M. Parental smoking and risk of childhood cancer: Hospital-based case-control study in Shiraz. East Mediterr Health J 2011;17:303-8.
- Chang JS, Selvin S, Metayer C, Crouse V, Golembesky A, Buffler PA. Parental smoking and the risk of childhood leukemia. Am J Epidemiol 2006;163:1091-100.
- Carozza SE, Puumala SE, Chow EJ, Fox EE, Horel S, Johnson KJ, et al. Parental educational attainment as an indicator of socioeconomic status and risk of childhood cancers. Br J Cancer 2010;103:136-42.
- Poole C, Greenland S, Luetters C, Kelsey JL, Mezei G. Socioeconomic status and childhood leukaemia: A review. Int J Epidemiol 2006;35:370-84.
- Pombo-de-Oliveira MS, Koifman S; Brazilian Collaborative Study Group of Infant Acute Leukemia. Infant acute leukemia and maternal exposures during pregnancy. Cancer Epidemiol Biomarkers Prev 2006;15:2336-41.
- Gholami A, Salarilak S, Hejazi S, Khalkhali HR. Parental risk factors of childhood acute leukemia: A case-control study. J Res Health Sci 2011;11:69-76.
- Brain JD, Kavet R, McCormick DL, Poole C, Silverman LB, Smith TJ, et al. Childhood leukemia: Electric and magnetic fields as possible risk factors. Environ Health Perspect 2003;111:962-70.
- Pelissari DM, Barbieri FE, Wünsch Filho V. Magnetic fields and acute lymphoblastic leukemia in children: A systematic review of case-control studies. Cad Saude Publica 2009;25 Suppl 3:S441-52.
- Colt JS, Blair A. Parental occupational exposures and risk of childhood cancer. Environ Health Perspect 1998;106 Suppl 3:909-25.
- Scélo G, Metayer C, Zhang L, Wiemels JL, Aldrich MC, Selvin S, et al. Household exposure to paint and petroleum solvents, chromosomal translocations, and the risk of childhood leukemia. Environ Health Perspect 2009;117:133-9.
- Feychting M, Plato N, Nise G, Ahlbom A. Paternal occupational exposures and childhood cancer. Environ Health Perspect 2001;109:193-6.
- Schüz J, Kaletsch U, Meinert R, Kaatsch P, Michaelis J. Risk of childhood leukemia and parental self-reported occupational exposure to chemicals, dusts, and fumes: Results from pooled analyses of German population-based case-control studies. Cancer Epidemiol Biomarkers Prev 2000;9:835-8.
- Alderton LE, Spector LG, Blair CK, Roesler M, Olshan AF, Robison LL, et al. Child and maternal household chemical exposure and the risk of acute leukemia in children with Down's syndrome: A report from the Children's Oncology Group. Am J Epidemiol 2006;164:212-21.
- Freedman DM, Stewart P, Kleinerman RA, Wacholder S, Hatch EE, Tarone RE, et al. Household solvent exposures and childhood acute lymphoblastic leukemia. Am J Public Health 2001;91:564-7.
- Jurewicz J, Hanke W. Exposure to pesticides and childhood cancer risk: Has there been any progress in epidemiological studies? Int J Occup Med Environ Health 2006;19:152-69.
- Swensen AR, Ross JA, Shu XO, Reaman GH, Steinbuch M, Robison LL. Pet ownership and childhood acute leukemia (USA and Canada). Cancer Causes Control 2001;12:301-3.
- Hassanzadeh J, Mohammadi R, Rajaeefard AR, Bordbar MR, Karimi M. Maternal and prenatal risk factors for childhood leukemia in southern of Iran. Iran Red Crescent Med J 2011;13:398-403.
- Linabery AM, Jurek AM, Duval S, Ross JA. The association between atopy and childhood/adolescent leukemia: A meta-analysis. Am J Epidemiol 2010;171:749-64.

| Figure 1:Ratio of different malignancies in Southern Iran

| Figure 2:Ratio oIncidence rate of cancers per million in children from 2011 to 2013
References
- Parkin DM, Stiller CA, Draper GJ, Bieber CA. The international incidence of childhood cancer. Int J Cancer 1988;42:511-20.
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917.
- Alderfer MA, Cnaan A, Annunziato RA, Kazak AE. Patterns of posttraumatic stress symptoms in parents of childhood cancer survivors. J Fam Psychol 2005;19:430-40.
- Luo W, Lane R, Stobart K, Morrison H, Schanzer D, Barr R, et al. The medical care cost of childhood and adolescent cancer in Manitoba, 1990-1995. Chronic Dis Can 2002;23:83-90.
- Asselin BL. Epidemiology of childhood and adolescent cancer. 19th ed. Philadelphia: Lippincott Williams & Wilkins; 2011. p. 1725-8.
- Savitz DA, Chen JH. Parental occupation and childhood cancer: Review of epidemiologic studies. Environ Health Perspect 1990;88:325-37.
- Westbom L, Aberg A, Källén B. Childhood malignancy and maternal diabetes or other auto-immune disease during pregnancy. Br J Cancer 2002;86:1078-80.
- Chow WH, Linet MS, Liff JM, Greenberg RS. Cancers in children. In: Schottenfeld D, Fraumeni JF Jr., editors. Cancer Epidemiology and Prevention. 2nd ed. New York: Oxford University Press; 1996. p. 1331-69.
- Namehnews.ir. Prevalence of Leukemia in Children. Available from: http://namehnews.ir/fa/news/171764.
- Fararunews. Toxic Small Particles Reach the Capital. Available from: http://www.fararu.com/fa/news/206747.
- ;IRNA. Parasites in Iran: Yes or No? News Code: (4201730) 81292960. Available from: http://www.irna.ir.
- Siegel DA, King J, Tai E, Buchanan N, Ajani UA, Li J. Cancer incidence rates and trends among children and adolescents in the United States, 2001-2009. Pediatrics 2014;134:e945-55.
- de Camargo B, de Oliveira Santos M, Rebelo MS, de Souza Reis R, Ferman S, Noronha CP, et al. Cancer incidence among children and adolescents in Brazil:First report of 14 population-based cancer registries. Int J Cancer 2010;126:715-20.
- Lacour B, Guyot-Goubin A, Guissou S, Bellec S, Désandes E, Clavel J. Incidence of childhood cancer in France: National Children Cancer Registries, 2000-2004. Eur J Cancer Prev 2010;19:173-81.
- Rivera-Luna R, Correa-González C, Altamirano-Alvarez E, Sánchez-Zubieta F, Cárdenas-Cardós R, Escamilla-Asian G, et al. Incidence of childhood cancer among Mexican children registered under a public medical insurance program. Int J Cancer 2013;132:1646-50.
- Swaminathan R, Rama R, Shanta V. Childhood cancers in Chennai, India, 1990-2001: Incidence and survival. Int J Cancer 2008;122:2607-11.
- Moradi A, Semnani S, Roshandel G, Mirbehbehani N, Keshtkar A, Aarabi M, et al. Incidence of childhood cancers in Golestan Province of Iran. Iran J Pediatr 2010;20:335-42.
- Mousavi SM, Pourfeizi A, Dastgiri S. Childhood cancer in Iran. J Pediatr Hematol Oncol 2010;32:376-82.
- ;Edraki M, Rambod M. Parental smoking and risk of childhood cancer: Hospital-based case-control study in Shiraz. East Mediterr Health J 2011;17:303-8.
- Chang JS, Selvin S, Metayer C, Crouse V, Golembesky A, Buffler PA. Parental smoking and the risk of childhood leukemia. Am J Epidemiol 2006;163:1091-100.
- Carozza SE, Puumala SE, Chow EJ, Fox EE, Horel S, Johnson KJ, et al. Parental educational attainment as an indicator of socioeconomic status and risk of childhood cancers. Br J Cancer 2010;103:136-42.
- Poole C, Greenland S, Luetters C, Kelsey JL, Mezei G. Socioeconomic status and childhood leukaemia: A review. Int J Epidemiol 2006;35:370-84.
- Pombo-de-Oliveira MS, Koifman S; Brazilian Collaborative Study Group of Infant Acute Leukemia. Infant acute leukemia and maternal exposures during pregnancy. Cancer Epidemiol Biomarkers Prev 2006;15:2336-41.
- Gholami A, Salarilak S, Hejazi S, Khalkhali HR. Parental risk factors of childhood acute leukemia: A case-control study. J Res Health Sci 2011;11:69-76.
- Brain JD, Kavet R, McCormick DL, Poole C, Silverman LB, Smith TJ, et al. Childhood leukemia: Electric and magnetic fields as possible risk factors. Environ Health Perspect 2003;111:962-70.
- Pelissari DM, Barbieri FE, Wünsch Filho V. Magnetic fields and acute lymphoblastic leukemia in children: A systematic review of case-control studies. Cad Saude Publica 2009;25 Suppl 3:S441-52.
- Colt JS, Blair A. Parental occupational exposures and risk of childhood cancer. Environ Health Perspect 1998;106 Suppl 3:909-25.
- Scélo G, Metayer C, Zhang L, Wiemels JL, Aldrich MC, Selvin S, et al. Household exposure to paint and petroleum solvents, chromosomal translocations, and the risk of childhood leukemia. Environ Health Perspect 2009;117:133-9.
- Feychting M, Plato N, Nise G, Ahlbom A. Paternal occupational exposures and childhood cancer. Environ Health Perspect 2001;109:193-6.
- Schüz J, Kaletsch U, Meinert R, Kaatsch P, Michaelis J. Risk of childhood leukemia and parental self-reported occupational exposure to chemicals, dusts, and fumes: Results from pooled analyses of German population-based case-control studies. Cancer Epidemiol Biomarkers Prev 2000;9:835-8.
- Alderton LE, Spector LG, Blair CK, Roesler M, Olshan AF, Robison LL, et al. Child and maternal household chemical exposure and the risk of acute leukemia in children with Down's syndrome: A report from the Children's Oncology Group. Am J Epidemiol 2006;164:212-21.
- Freedman DM, Stewart P, Kleinerman RA, Wacholder S, Hatch EE, Tarone RE, et al. Household solvent exposures and childhood acute lymphoblastic leukemia. Am J Public Health 2001;91:564-7.
- Jurewicz J, Hanke W. Exposure to pesticides and childhood cancer risk: Has there been any progress in epidemiological studies? Int J Occup Med Environ Health 2006;19:152-69.
- Swensen AR, Ross JA, Shu XO, Reaman GH, Steinbuch M, Robison LL. Pet ownership and childhood acute leukemia (USA and Canada). Cancer Causes Control 2001;12:301-3.
- Hassanzadeh J, Mohammadi R, Rajaeefard AR, Bordbar MR, Karimi M. Maternal and prenatal risk factors for childhood leukemia in southern of Iran. Iran Red Crescent Med J 2011;13:398-403.
- Linabery AM, Jurek AM, Duval S, Ross JA. The association between atopy and childhood/adolescent leukemia: A meta-analysis. Am J Epidemiol 2010;171:749-64.


 PDF
PDF  Views
Views  Share
Share

