A Study on Utilization and Evaluation of Antiemetics in Chemotherapy induced Nausea and Vomiting
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(03): 334-339
DOI: DOI: 10.4103/ijmpo.ijmpo_116_17
Abstract
Purpose: Chemotherapy-induced nausea and vomiting (CINV) are the major adverse effects of cancer chemotherapy. The objectives of this study are to evaluate the utilization of antiemetics in CINV and to assess the emetogenicity of chemotherapy and to investigate the incidence of acute and delayed CINV. Methods: A prospective observational study was carried out in patients undergoing chemotherapy. A suitable data collection form was designed to collect data regarding patient's demographics, cancer type, chemotherapy regimen, antiemetic prescribed, and incidence of CINV according to the standard methods utilizing morrow assessment of nausea and emesis form. Results: Among 200 patients enrolled in the study, with age range of 18–83 (52 ± 11.65; mean ± standard deviation) of both sexes (44% of male and 56% of female), 38.5% of patients received highly emetogenic chemotherapy and 46.5% received moderate emetogenic chemotherapy. Among the patients, 88% received 5HT3-RA in combination with corticosteroid (99%) and NK1-RA (40.5%). Despite the administration of antiemetic, the incidence of acute and delayed nausea after chemotherapy treatment was reported by 54% and 15.5%, respectively. The comparable figures for acute and delayed vomiting were 36.5% and 14.5%. Conclusion: The incidence of CINV among the patients was relatively high and it indicates that more attention is needed for the treatment of both acute and delayed CINV. It also gives an idea for implementation of more efficient antiemesis guideline in the clinical practice.
Publication History
Article published online:
04 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Purpose:
Chemotherapy-induced nausea and vomiting (CINV) are the major adverse effects of cancer chemotherapy. The objectives of this study are to evaluate the utilization of antiemetics in CINV and to assess the emetogenicity of chemotherapy and to investigate the incidence of acute and delayed CINV.
Methods:
A prospective observational study was carried out in patients undergoing chemotherapy. A suitable data collection form was designed to collect data regarding patient's demographics, cancer type, chemotherapy regimen, antiemetic prescribed, and incidence of CINV according to the standard methods utilizing morrow assessment of nausea and emesis form.
Results:
Among 200 patients enrolled in the study, with age range of 18–83 (52 ± 11.65; mean ± standard deviation) of both sexes (44% of male and 56% of female), 38.5% of patients received highly emetogenic chemotherapy and 46.5% received moderate emetogenic chemotherapy. Among the patients, 88% received 5HT3-RA in combination with corticosteroid (99%) and NK1-RA (40.5%). Despite the administration of antiemetic, the incidence of acute and delayed nausea after chemotherapy treatment was reported by 54% and 15.5%, respectively. The comparable figures for acute and delayed vomiting were 36.5% and 14.5%.
Conclusion:
The incidence of CINV among the patients was relatively high and it indicates that more attention is needed for the treatment of both acute and delayed CINV. It also gives an idea for implementation of more efficient antiemesis guideline in the clinical practice.
Introduction
Cancer is one of the leading causes of morbidity and mortality worldwide. According to world cancer fund, one-third of cancer cases in economically developed countries are due to physical inactivity, excess alcohol consumption, smoking, obesity, and poor nutrition. In case of developing countries like India, life style changes and environmental factors are major causes of cancer. According to the American Cancer Society, about 1688,780 new cancer cases were expected to be diagnosed and 600,920 cancer deaths in 2017.[1] Based on cancer scenario in India the incidence of cancer is 70–90/100,000 population and prevalence of cancer is found to be around 2.5 million with over 800,000 new cases and 550,000 deaths occurring each year. About 6% of all deaths in India are due to cancers, which contribute to 8% of global cancer mortality.[2]
Chemotherapy is the main-stay of treatment in patients with advanced malignant disease, which is incurable by local surgery or radiotherapy.[3] Although there are different targeted therapies have been approved for cancer therapy, these therapies may compliment rather than replace chemotherapy. However, chemotherapy improves survival it has its own toxicity and side effects. The most common of which are nausea and vomiting, diarrhea, alopecia, mucositis, and myelosuppression. Among which nausea and vomiting are the frequently observed side effect of chemotherapy. Chemotherapy-induced nausea and vomiting (CINV) also leads to fatigue, muscle strain, esophageal tears, metabolic imbalance, lowered cognitive function, and increased anxiety or depression.[4]
Three different types of CINV have been identified; namely, Acute, delayed, and anticipatory. Acute emesis begins within 1–2 h of treatment and peaks in the first 4–6 h. Delayed emesis occurs more than 24 h after treatment, peaks at 48–72 h and then subsides over 2–3 days.[5] Anticipatory emesis occurs in patients who have developed significant CINV during previous cycles of therapy. Acute CINV is often associated with an increase in plasma serotonin concentrations for the most emetogenic agent while delayed and anticipatory vomiting seem to be mediated by serotonin independent pathways.[5] The patient risk for CINV is influenced by the emetogenicity of the chemotherapeutic agent.
The chemotherapeutic agents are classified into four emetic risk categories. It includes high (>90% of frequency of emesis), moderate (30%–90%), low (10%–30%), and minimal (<10 href="https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5686978/#ref5" rid="ref5" class=" bibr popnode" role="button" aria-expanded="false" aria-haspopup="true" xss=removed>5] The guidelines to prevent CINV includes the American Society of Clinical Oncology,[6] the Multinational Association for Supportive Care in Cancer[7] and National Comprehensive Cancer Network (NCCN). The principles of emesis control for cancer patient from NCCN include; (1) the goal of treatment is to prevent nausea/vomiting. (2) The risk period for chemotherapy induced nausea and vomiting with highly emetogenic chemotherapy (HEC) and moderate emetogenic chemotherapy (MEC) is at least 3 days and patients should be protected for the entire risk period. (3) Oral and intravenous (IV) 5-HT3 antagonists have equivalent efficacy when used at the appropriate doses. (4) choice of antiemetic(s) used should be based on emetic risk of chemotherapy, a patient's prior antiemetic experience, and other patient factors; and (5) for multi-drug regimens, select antiemetic therapy based on the drug with the HEC and consider using an H2 blocker or proton pump inhibitor to prevent dyspepsia, which can mimic nausea.
Although there are many observational studies to implement antiemesis guidelines in practice, the adherence to guidelines in preventing CINV still remains as a main issue. The current study helps to evaluate the utilization of antiemetics in CINV, to assess the emetogenicity of chemotherapeutic agents and also the incidence of CINV.
Methods
It was a prospective study carried out in patients who were admitted in the Department of Oncology at a Teaching Hospital. The study was conducted from October 2016 to March 2017. Hospitalized patients above 18 years of either gender receiving chemotherapy was included in the study and patients with brain metastases, radiation-induced nausea and vomiting were excluded from the study. This study was approved by the Central Ethics Committee of the institution (enclosed), and informed consent form was obtained from all the participants.
A suitable data collection form was designed to collect and document data. It had a provision for recording information related to demographic details of the patient, cancer/treatment informations. A separate section was provided to record the details of the medications the patients were on, at the time and during the study.
To find out the incidence of chemotherapy-induced nausea and vomiting morrow assessment of nausea and emesis (MANE) form[8] was used. The MANE form involved questionnaire about the incidence of CINV, whether before or after the last chemotherapy treatment, the duration that patient experiences CINV in hours, and the description of CINV at its worst (very mild, mild, moderate, severe, very severe, and intolerable), the MANE form also involved questionnaire about the usefulness of antiemetic taking by patients which scored as (very useful, somewhat useful, works a little, or does not seem to help).
The chemotherapeutic regimens given were assessed based on their emetogenic potential and then classified them into high, moderate, low, and minimal using the standard NCCN guidelines.
Results
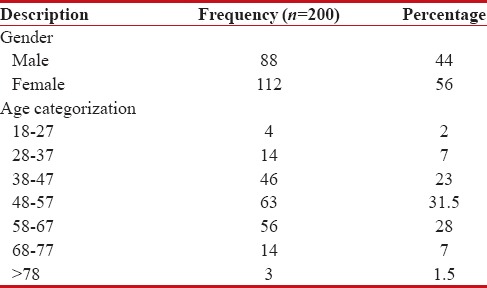
The questionnaire was completed for 200 cancer patients receiving chemotherapy. Among 200 cancer patients participated in the study, majority 112 (56%) were found to be female and remaining 88 (44%) were male. The mean age was found to be 52 ± 11.65 (range: 18–83) years [Table 1].
Table 1
Demographic distribution of the subjects
|
The distribution of cancer among study population shows breast cancer in 51 (25.5%) patients, and it was found to be the most common cancer among study population [Figure 1].
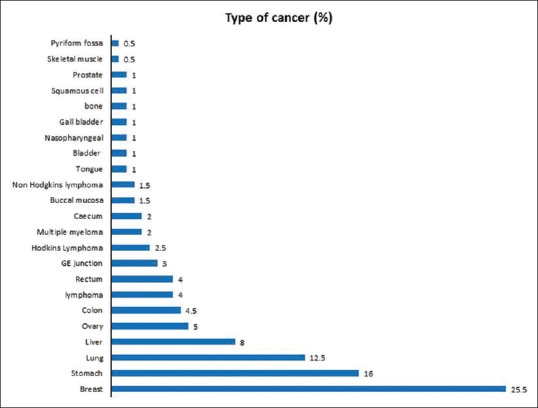
| Figure 1:Pattern of clinical conditions among the subjects
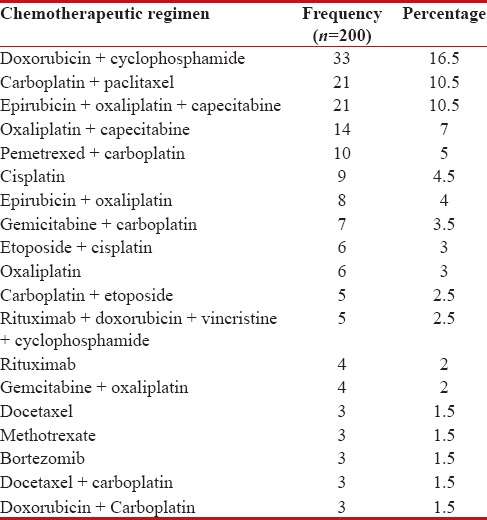
Table 2 shows that among study population most commonly used chemotherapy regimen is Doxorubicin and Cyclophosphamide 33 (16.5%).
Table 2
Pattern of most commonly used chemotherapy regimen in cancer patients
|
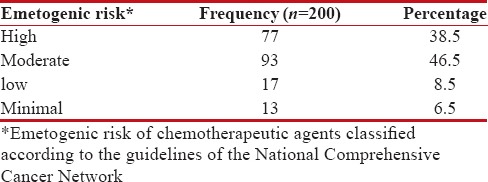
Emetogenic risk of chemotherapeutic agents
Among 200 patients, 77 (38.5%) received HEC, followed by 93 (46.5%) received moderate, 17 (8.5%) received low and 13 (6.5%) patients received minimal. Table 3 shows the frequency of chemotherapy prescribed with their emetogenic risk.
Table 3
The frequency of chemotherapy prescribed with their emetogenic risk
|
Prescribing pattern of antiemetics
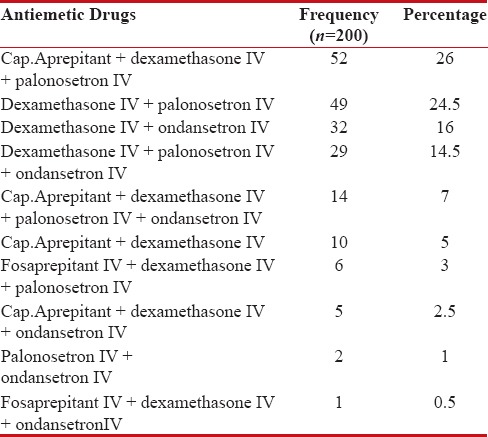
Antiemetic therapy used was consistent with the standard NCCN guidelines of antiemesis. Nearly, all the patients (88%) received 5HT3-RA in combination with corticosteroid (99%) and NK1-RA (40.5%). The frequency of antiemetics used is shown in Table 4.
Table 4
Frequency of antiemetics used
|
Incidence of nausea postchemotherapy treatment
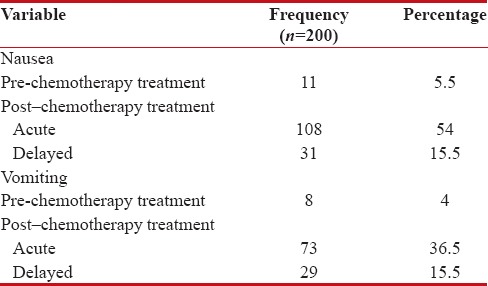
During the study, the total incidence of nausea postchemotherapy treatment was 139 (69.5%). The incidence was more in females 90 (45%) than males 49 (24.5%). Table 5 shows the incidence of nausea and vomiting pre- and post-chemotherapy treatment.
Table 5
The incidence of Nausea and Vomiting pre and post- chemotherapy treatment
|
Concerning the severity of nausea incident, postchemotherapy treatment was mild 65 (32.5%), moderate 46 (23%), very mild 20 (10%), followed by severe 8 (4%). Figure 2 demonstrates the severity of nausea incident postchemotherapy treatment.

| Figure 2:Severity of nausea incident postchemotherapy (%)
The time of incidence of nausea at the first 24 h from the administration of chemotherapy was found to be 108 (54%), which represents acute nausea and after 24 h was found to be 31 (15.5%), which represents delayed nausea. Incident time of nausea postchemotherapy treatment is shown in Figure 3.
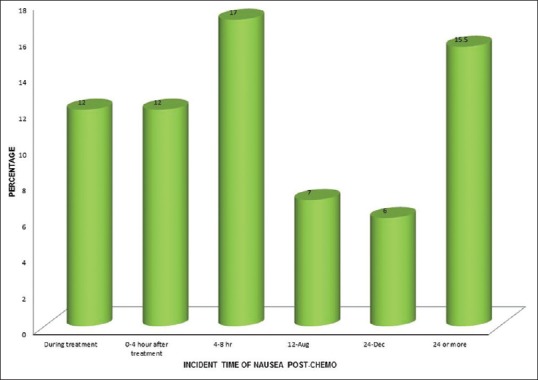
| Figure 3:Incident time of nausea postchemotherapy (%)
Incidence of nausea prechemotherapy treatment
The incidence of nausea before chemotherapy treatment was found to be 11 (5.5%), which represents anticipatory nausea. The severity of nausea incident prechemotherapy was moderate 9 (4.5%), very mild one (0.5%), and mild one (0.5%).
Incidence of vomiting postchemotherapy treatment
The total incidence of vomiting was 92 (46%) postchemotherapy treatment, in which females 59 (29.5%) showed more incidence vomiting than males 33 (16.5%). Concerning the severity of vomiting incident, postchemotherapy treatment was moderate 39 (19.5%), mild 34 (17%), very mild nine (4.5%), followed by severe nine (4.5%), and intolerable 1 (0.5%) is shown in Figure 4.
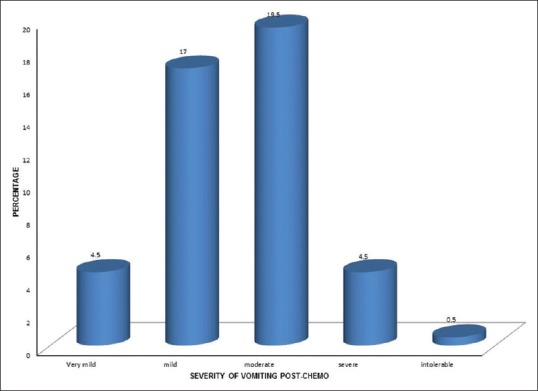
| Figure 4:Severity of vomiting incident postchemotherapy (%)
The time of incidence of vomiting at the first 24 h from the administration of chemotherapy was found to be 73 (36.5%), which represents acute vomiting, and after 24 h was found to be 29 (14.5%), which represents delayed vomiting. The incident time of vomiting postchemotherapy treatment is shown in Figure 5.
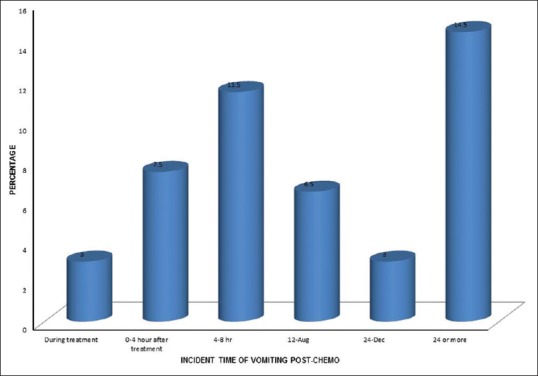
| Figure 5:Incident time of vomiting postchemotherapy (%)
Incidence of vomiting prechemotherapy treatment
The incidence of vomiting before chemotherapy treatment was found to be 8 (4%), which represents anticipatory vomiting. The severity of vomiting incidence prechemotherapy was moderate three (2.5%), followed by mild in three (1.5%), very mild in one (0.5%), and severe in one (0.5%) patients.
Usefulness of medication for chemotherapy induced nausea and vomiting for the last chemotherapy treatment
Out of 200 patients included in the study population, 198 (99%) patients received antiemetics treatment. Concerning the usefulness, 141 (70.5%) patients reported that antiemetic treatment was very useful, 51 (21.5%) reported somewhat useful, six (3%) reported works little, and one (0.5%) reported does not seem to help.
Usefulness of medication for CINV for the last chemotherapy treatment is shown in Figure 6.
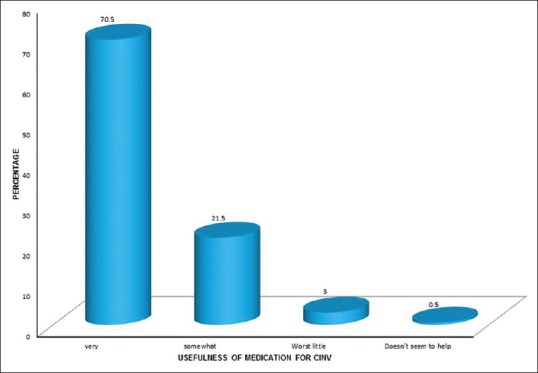
| Figure 6:Usefulness of medication for chemotherapy-induced nausea and vomiting for chemotherapy
Discussion
The present study was conducted to study the utilization and evaluation of antiemetics used in CINV, and to evaluate the incidence of CINV. Two hundred patients were included in the study. The prevalence of cancer is found to be more in females than males. These results were consistent with previously published studies in oncology.[9,10,11] In this study, the majority of patients were in the age group of 48–57 years and the mean age was found to be 52 ± 11.65 years. This indicates that middle-aged people have higher incidence of cancer. These findings correlate with other studies.[9,12,13]
The study showed that the most common cancer was found to be breast cancer.[4,9] The most commonly used chemotherapy regimen was doxorubicin and cyclophosphamide combination followed by carboplatin and paclitaxel, epirubicin, oxaliplatin, and capecitabine. Cisplatin was the most commonly used individual chemotherapy drug.[14,15] The current study assessed the emetogenicity of chemotherapeutic agents according to the NCCN guideline. It revealed that most of the subjects received MEC followed by HEC,[13] in contrary, HEC was prescribed more than MEC regimen.[12]
The present study showed that the antiemetic therapy used was consistent with the standard guidelines of NCCN. For acute and delayed emesis, the updated NCCN guideline recommends triple therapy with an NK1-RA (aprepitant or fosaprepitant), 5HT3-RA (palonosetron or ondansetron or granisetron), and corticosteroid (dexamethasone) for HEC regimens. In MEC regimens, double therapy of 5HT3-RA and dexamethasone is recommended with or without NK1-RA. In the case of low emetogenic chemotherapy, a monotherapy of dexamethasone or Metoclopramide or 5HT3-RA is recommended, and no prophylaxis antiemetic therapy is recommended for minimal emetogenic chemotherapy. The results of prescribing pattern of antiemetic showed that most of the subjects are prescribed with Cap. aprepitant, dexamethasone IV, and palonosetron IV followed by dexamethasone IV and palonosetron IV. Almost all the patients received 5HT3-RA in combination with corticosteroid and NK1-RA. The results were consistent with other findings.[12,13]
The findings of the current study showed that there was good adherence to the current antiemesis guideline. However, the incidence of CINV was high. Among the study population, the incidence of nausea postchemotherapy was 69.5% of which 54% for acute and 15.5% for delayed. The incidence of vomiting postchemotherapy was 46%, in which 36.5% for acute and 14.5% for delayed. Whereas in other study, the incidence of nausea postchemotherapy was found to be 65.38% and vomiting 50%.[12]
The time of incidence of delayed (>24 h) nausea and vomiting after chemotherapy was 15.5% and 14.5%, respectively. The severity of nausea and vomiting incidence postchemotherapy was found to be mild (32.5%) and moderate (19.5%), respectively.[12] These results indicate that CINV remains a major problem for cancer patients receiving chemotherapy and it also gives an idea about the need for more efficient implementation of guidelines of antiemesis in the clinical practice.
Patient satisfaction regarding the usefulness of antiemetic medication shows that 70.5% patients reported that antiemetic medication was very useful, 21.5% reported somewhat useful, 3% works a little and 0.5% reported that does not seem to help. Whereas in other study,[12] they found that 38.46% of patients reported that antiemetic treatment used was not useful and 61.51% reported as useful.
The current study showed that incidence of nausea and vomiting prechemotherapy treatment was found to be 5.5% and 4%, respectively. The results were found to be in contrast that the incidence of CINV prechemotherapy treatment was zero.[12]
Conclusion
The study evaluated the utilization of antiemetics in CINV and the incidence of nausea and vomiting in cancer patients receiving chemotherapy. The majority of the patients received HEC and MEC regimen of chemotherapy and the most commonly prescribed antiemetic regimen was triple therapy (5HT3-RA, corticosteorid, and NK1-RA) followed by double therapy (5HT3-RA and Corticosteroid). In conclusion, the incidence of CINV was relatively high, and it indicates that more attention is needed for the treatment of both acute and delayed CINV. It also gives an idea for implementation of more efficient antiemesis guideline in the clinical practice.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We are thankful to the authorities of Nitte University, Mangalore, India; Medical Superitendent, KS Hegde Charitable Hospital, Mangalore; Dr. Jayaram Shetty, Head, Department of Oncology, KS Hegde Charitable Hospital, Mangalore; Dr. C.S. Shasty, Principal, NGSM Institute of Pharmaceutical Sciences, Mangalore. Mr. Sanal Kumar, Statistician, Nitte University.
References
- American Cancer Society. Cancer Facts and Figures 2017. Atlanta: American Cancer Society; 2017.
- Bushan K, Cancer Scenario in India. Daily Excelsior.com, 2014. Available from: http://www.dailyexcelsior.com/cancer-scenario-india/. [Last accessed on 2017 Apr 28].
- Sharma R, Tobin P, Clarke SJ. Management of chemotherapy-induced nausea, vomiting, oral mucositis, and diarrhoea. Lancet Oncol 2005;6:93-102.
- Roscoe JA, Morrow GR, Hickok JT, Stern RM. Nausea and vomiting remain a significant clinical problem: Trends over time in controlling chemotherapy-induced nausea and vomiting in 1413 patients treated in community clinical practices. J Pain Symptom Manage 2000;20:113-21.
- Manson E, Routledge PA. Nausea and vomiting. In: Walker R, Whittlesia C, editors. Clinical Pharmacy and Therapeutics. 5th ed. China: Churchill Livingstone, Elsevier; 2012. p. 535-44.
- Jordan K, Sippel C, Schmoll HJ. Guidelines for antiemetic treatment of chemotherapy-induced nausea and vomiting: Past, present, and future recommendations. Oncologist 2007;12:1143-50.
- Prevention of chemotherapy-and radiotherapy-induced emesis: Results of Perugia Consensus Conference. Antiemetic Subcommittee of the Multinational Association of Supportive Care in Cancer (MASCC). Ann Oncol 1998;9:811-9.
- Morrow GR. A patient report measure for the quantification of chemotherapy induced nausea and emesis: Psychometric properties of the Morrow assessment of nausea and emesis (MANE). Br J Cancer Suppl 1992;19:S72-4.
- Hilarius DL, Kloeg PH, van der Wall E, van den Heuvel JJ, Gundy CM, Aaronson NK. Chemotherapy-induced nausea and vomiting in daily clinical practice: A community hospital-based study. Support Care Cancer 2012;20:107-17.
- Gilmore JW, Peacock NW, Gu A, Szabo S, Rammage M, Sharpe J, et al. Antiemetic guideline consistency and incidence of chemotherapy-induced nausea and vomiting in US community oncology practice: INSPIRE Study. J Oncol Pract 2014;10:68-74.
- Eisenberg P, Vadillo JF, Zamora R, Charu V, Hajdenberg J, Cartmell A, et al. Improved prevention of moderately emetogenic chemotherapy induced nausea and vomiting with palonosetron, a pharmacologically novel 5-HT3 receptor antagonist. Am Cancer Soc 2003;98:2473-82.
- Sahib AS, Hamdan SJ, Mohammed IH, Khefi AA. Prescribing pattern of antiemetics in the treatment of chemotherapy induced nausea and vomiting. World J Pharm Res 2014;3:3639-54.
- Zong X, Zhang J, Ji X, Gao J, Ji J. Patterns of antiemetic prophylaxis for chemotherapy-induced nausea and vomiting in China. Chin J Cancer Res 2016;28:168-79.
- Grunberg SM, Deuson RR, Mavros P, Geling O, Hansen M, Cruciani G, et al. Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Am Cancer Soc 2004;100:2261-8.
- de Boer-Dennert M, de Wit R, Schmitz PI, Djontono J, v Beurden V, Stoter G, et al. Patient perceptions of the side-effects of chemotherapy: The influence of 5HT3 antagonists. Br J Cancer 1997;76:1055-61.

| Figure 1:Pattern of clinical conditions among the subjects

| Figure 2:Severity of nausea incident postchemotherapy (%)

| Figure 3:Incident time of nausea postchemotherapy (%)

| Figure 4:Severity of vomiting incident postchemotherapy (%)

| Figure 5:Incident time of vomiting postchemotherapy (%)

| Figure 6:Usefulness of medication for chemotherapy-induced nausea and vomiting for chemotherapy
References
- American Cancer Society. Cancer Facts and Figures 2017. Atlanta: American Cancer Society; 2017.
- Bushan K, Cancer Scenario in India. Daily Excelsior.com, 2014. Available from: http://www.dailyexcelsior.com/cancer-scenario-india/. [Last accessed on 2017 Apr 28].
- Sharma R, Tobin P, Clarke SJ. Management of chemotherapy-induced nausea, vomiting, oral mucositis, and diarrhoea. Lancet Oncol 2005;6:93-102.
- Roscoe JA, Morrow GR, Hickok JT, Stern RM. Nausea and vomiting remain a significant clinical problem: Trends over time in controlling chemotherapy-induced nausea and vomiting in 1413 patients treated in community clinical practices. J Pain Symptom Manage 2000;20:113-21.
- Manson E, Routledge PA. Nausea and vomiting. In: Walker R, Whittlesia C, editors. Clinical Pharmacy and Therapeutics. 5th ed. China: Churchill Livingstone, Elsevier; 2012. p. 535-44.
- Jordan K, Sippel C, Schmoll HJ. Guidelines for antiemetic treatment of chemotherapy-induced nausea and vomiting: Past, present, and future recommendations. Oncologist 2007;12:1143-50.
- Prevention of chemotherapy-and radiotherapy-induced emesis: Results of Perugia Consensus Conference. Antiemetic Subcommittee of the Multinational Association of Supportive Care in Cancer (MASCC). Ann Oncol 1998;9:811-9.
- Morrow GR. A patient report measure for the quantification of chemotherapy induced nausea and emesis: Psychometric properties of the Morrow assessment of nausea and emesis (MANE). Br J Cancer Suppl 1992;19:S72-4.
- Hilarius DL, Kloeg PH, van der Wall E, van den Heuvel JJ, Gundy CM, Aaronson NK. Chemotherapy-induced nausea and vomiting in daily clinical practice: A community hospital-based study. Support Care Cancer 2012;20:107-17.
- Gilmore JW, Peacock NW, Gu A, Szabo S, Rammage M, Sharpe J, et al. Antiemetic guideline consistency and incidence of chemotherapy-induced nausea and vomiting in US community oncology practice: INSPIRE Study. J Oncol Pract 2014;10:68-74.
- Eisenberg P, Vadillo JF, Zamora R, Charu V, Hajdenberg J, Cartmell A, et al. Improved prevention of moderately emetogenic chemotherapy induced nausea and vomiting with palonosetron, a pharmacologically novel 5-HT3 receptor antagonist. Am Cancer Soc 2003;98:2473-82.
- Sahib AS, Hamdan SJ, Mohammed IH, Khefi AA. Prescribing pattern of antiemetics in the treatment of chemotherapy induced nausea and vomiting. World J Pharm Res 2014;3:3639-54.
- Zong X, Zhang J, Ji X, Gao J, Ji J. Patterns of antiemetic prophylaxis for chemotherapy-induced nausea and vomiting in China. Chin J Cancer Res 2016;28:168-79.
- Grunberg SM, Deuson RR, Mavros P, Geling O, Hansen M, Cruciani G, et al. Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Am Cancer Soc 2004;100:2261-8.
- de Boer-Dennert M, de Wit R, Schmitz PI, Djontono J, v Beurden V, Stoter G, et al. Patient perceptions of the side-effects of chemotherapy: The influence of 5HT3 antagonists. Br J Cancer 1997;76:1055-61.


 PDF
PDF  Views
Views  Share
Share

