A study to correlate histopathology, biochemical marker and immunohistochemical expression of sex-steroid receptors in prostatic growth
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2014; 35(01): 40-43
DOI: DOI: 10.4103/0971-5851.133719
Abstract
Abstract
Prostate gland is a fibromusculoglandular structure situated at the neck of urinary bladder. So, enlargement or growth of prostate due to nodular hyperplasia (NHP) or prostatic intraepithelial neoplasia (PIN) or adenocarcinoma may give rise to bladder outlet obstruction. Malignant growth i.e., PIN or adenocarcinoma cases are associated with increased blood level of prostate-specific antigen (PSA) and increased expression of different sex-steroid receptors because the growth is dependent on the interactions of androgen, progesterone and estrogen. The aim of our study is to correlate the histopathology, PSA levels and expression of different sex-steroid receptors by immunohistochemistry in different prostatic growth lesions. Among the total 50 cases received, inclusive of transurethral resection of prostate (TURP), transrectal ultrasound-guided biopsy and radical prostatectomy, 34 cases were diagnosed as NHP, 4 cases as PIN and 12 cases as adenocarcinoma histopathologically. Serum PSA values above 10 ng/ml were seen in 2 cases of PIN and 11 cases of adenocarcinoma and none of NHP. Estrogen receptor (ER) () expressions were negative in all cases. Progesterone receptor (PR) expressions were strongly positive in 35%-cases of both NHP and adenocarcinoma, whereas androgen receptor (AR) expressions were strong among all cases of adenocarcinoma and only in four cases of NHP. By observing these findings it can be suggested that antiandrogen and antiprogesterone therapy simultaneously will do better than antiandrogen alone in treating prostatic growth lesions.
Keywords
Androgen receptor - immunohistochemistry - progesterone receptor - prostatic growth - prostate-specific antigenPublication History
Article published online:
19 July 2021
© 2014. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Prostate gland is a fibromusculoglandular structure situated at the neck of urinary bladder. So, enlargement or growth of prostate due to nodular hyperplasia (NHP) or prostatic intraepithelial neoplasia (PIN) or adenocarcinoma may give rise to bladder outlet obstruction. Malignant growth i.e., PIN or adenocarcinoma cases are associated with increased blood level of prostate-specific antigen (PSA) and increased expression of different sex-steroid receptors because the growth is dependent on the interactions of androgen, progesterone and estrogen. The aim of our study is to correlate the histopathology, PSA levels and expression of different sex-steroid receptors by immunohistochemistry in different prostatic growth lesions. Among the total 50 cases received, inclusive of transurethral resection of prostate (TURP), transrectal ultrasound-guided biopsy and radical prostatectomy, 34 cases were diagnosed as NHP, 4 cases as PIN and 12 cases as adenocarcinoma histopathologically. Serum PSA values above 10 ng/ml were seen in 2 cases of PIN and 11 cases of adenocarcinoma and none of NHP. Estrogen receptor (ER) () expressions were negative in all cases. Progesterone receptor (PR) expressions were strongly positive in 35%-cases of both NHP and adenocarcinoma, whereas androgen receptor (AR) expressions were strong among all cases of adenocarcinoma and only in four cases of NHP. By observing these findings it can be suggested that antiandrogen and antiprogesterone therapy simultaneously will do better than antiandrogen alone in treating prostatic growth lesions.
INTRODUCTION
Prostate is a fibromusculoglandular organ encircling the neck of the bladder and male urethra, weighing up to 20 g in normal adults. Anatomically, it has four distinct zones – peripheral, central, transitional and anterior fibromuscular stroma. Hyperplasia arises from the transitional zone and carcinoma from the peripheral zone.[1]
Prostatic enlargement or growth most commonly occurs due to nodular hyperplasia or due to neoplasia like adenocarcinoma of tubuloalveolar glands and also its precursor lesions – prostatic intraepithelial lesion (PIN). All these conditions arise in males after 50 years of age and are due to the effect of androgen released from testis. All these conditions cause bladder outlet obstruction giving rise to different clinical features like dysuria, retention of urine and eneuresis. Low-back pain due to vertebral metastasis is common in the late stage of prostatic carcinoma. This malignancy, though uncommon in Asian countries, the incidence is increasing in our country 1% every year. The incidence of PIN is even higher, approximately 70%-above the 70 years of age.[2] To detect early this commonly occurring malignancy in men, screening tests are considered after the age of 40 years, which include digital rectal examination (DRE), transrectal ultrasonography (TRUS) and estimation of prostate-specific antigen (PSA) in serum.[3] PSA is secreted by luminal epithelial cells of prostatic glands. Serum PSA level above 4 ng/ml is noted in most cases of adenocarcinoma of prostate and if it is increased more than 0.15 per unit volume of prostate (PSA density), it is indicative of adenocarcinoma of prostate.[4]
Androgen receptor (AR) also known as NR3C4 is a type of nuclear receptor which is activated by binding of either of the androgenic hormones. The ARs are closely related to progesterone receptor (PR) and progestins in higher dose can block the ARs.
AR remains important in the development and progression of prostatic carcinoma and AR expression is maintained throughout prostate carcinoma progression and the majority of androgen-independent or hormone-refractory prostate carcinoma express AR.[5] Estrogen receptor (ER) and estrogen are implicated in prostatic carcinogenesis and tumor progression.[6] The PRs are also nuclear receptors and progressive emergence of PRs during tumor progression obviously reflects the ability of metastatic and androgen-insensitive tumors to use estrogens through an ER-α-mediated pathway. So, antiestrogens and SERMs can suppress progression of prostatic carcinoma.[7]
Here, our objective of this study was to correlate the histopathology of prostatic tissues resected due to different growth with PSA level in serum and expression of AR, ER and PR by immunohistochemistry (IHC).
MATERIALS AND METHODS
The study was done in the Departments of Pathology and Urology of IPGMER, Kolkata, during the period of July 2008 to August 2010. A total of 50 cases were collected which were diagnosed in Urology Dpt. as cases of prostatic growth. Among them, 35 were Transurethral resection of prostate (TURP) specimens with clinicoradiological diagnosis of NHP, 05 were trasurethral ultrasonography–guided biopsy (TRUS-BX) specimens and remaining 10 specimens were of radical prostatectomy specimens with clinicoradiologic and biochemical markers favoring prostatic adenocarcinoma.
Gross details of sent specimens along with clinical, radiological and serum PSA level of all patients were noted. Hematoxylin and Eosin (HandE)-stained sections were prepared for routine histopathological evaluation to see nature of growth including Gleason scoring in cases of carcinoma. Poly-l-Lysine-coated slides were used for immunohistochemical staining for AR, ER and PR. The different reagents for IHC were provided by Biogenex including positive control. AR, ER, and PR expressions were assessed by ‘Quick-Score’ as applied in breast tissue.
Statistical analysis was done by using STATISTICA data analysis software version 6.0 [Tulsa, Oklahoma; Statsoft, Inc., 2001] considering a ‘P’ value below 0.05 as significant.
RESULTS AND ANALYSIS
We studied total fifty (50) cases including TURP, TRUS-BX and specimens of radical prostatectomy of which 34 cases were diagnosed as NHP (68%), 4 cases as PIN (8%) and12 cases as prostatic adenocarcinoma (24%) as per routine histopathology [Figure 1].
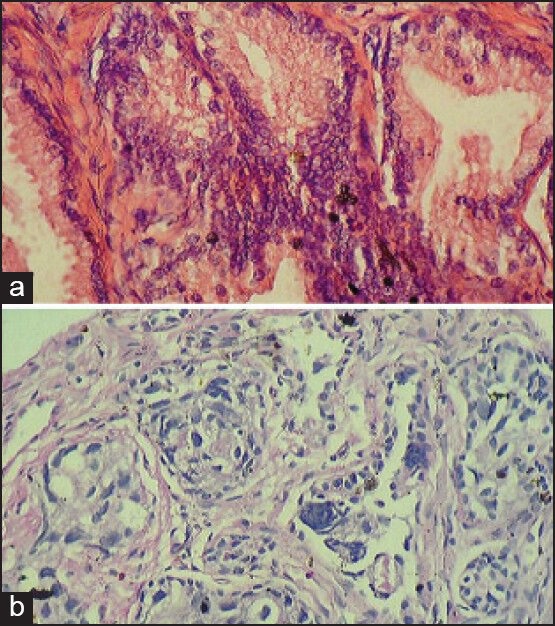
| Figure 1:(a) Histopathology of prostatic intraepithelial neoplasia (H/E ×400). (b) Histopathology of prostatic adenocarcinoma (H/E ×400)
The mean age was 68.66 years. Among all only 7 patients were under 60 years, 22 were between 61 and 70 years, 17 were between 71 and 80 years and 4 patients were above 80 years of age. Among the 34 cases of NHP, 6 cases were under 60 years, whereas 3 cases were above 80 years. All cases of PIN were noted above 70 years of age. Among the carcinoma cases 7 cases were seen in between 71 and 80 years of age, 3 cases were between 61 and 70 years, and one case each below 60 years and above 80 years.
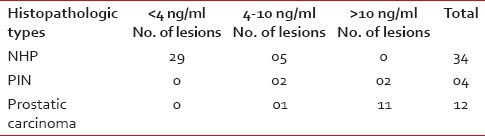
PSA values >10 ng/ml were noted in 11 cases of carcinoma and among which 7 cases had >100 ng/ml. Twenty-nine cases of NHP subjects had PSA values <4 href="https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4080662/table/T1/" target="table" class="fig-table-link figpopup" rid-figpopup="T1" rid-ob="ob-T1" co-legend-rid="" xss=removed>Table 1]. Higher PSA values ranging between 55.6and 1011.3 were observed in nine cases of prostatic adenocarcinoma with a high Gleason score between 8 and 10, whereas it was in the range of 12.13-57 in cases with score 7 (2 cases) and one case with score 6 had PSA value 8.2 [Table 2].
Table 1
(PSA values in different prostatic growth)
|
Table 2
PSA values in different Gleason scores of prostatic carcinoma

|
ER expressions analyzed by immunostaining were negative in all cases. PR expression was strongly positive in 10 cases of NHP, 1 case of PIN and 4 cases of Prostatic adenocarcinoma. The remaining cases were negative [Figure 2].
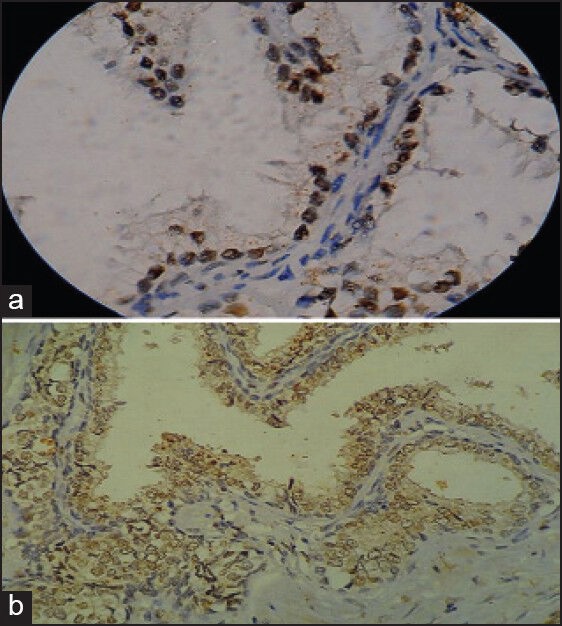
| Figure 2:(a) Nodular hyperplasia of prostate (monoclonal antibody against progesterone receptors ×1000). (b) Prostatic adenocarcinoma (monoclonal antibody against progesterone receptors ×400)
AR expression was strongly positive in 4 cases of NHP, 2 cases of PIN and all 12 cases of adenocarcinoma. It was weakly positive in 30 cases of NHP and 2 cases of PIN. None was negative [Table 3 and Figure 3].
Table 3
PR and AR expression in different prostatic growths

|
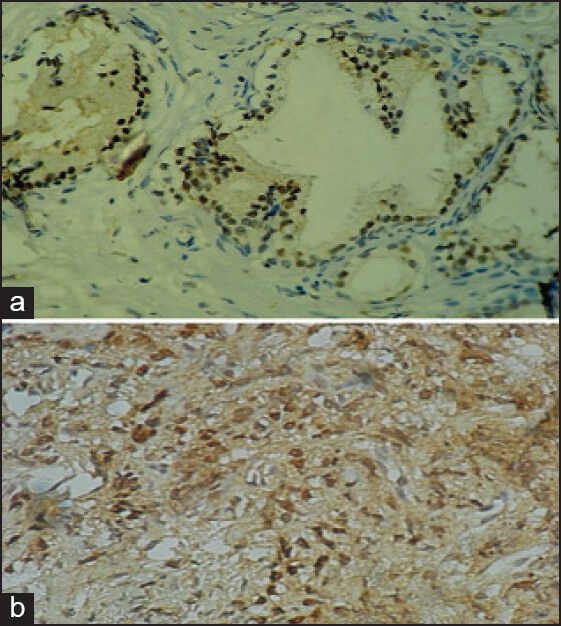
| Figure 3:(a) Nodular hyperplasia of prostate (monoclonal antibody against androgen receptors ×400). (b) Prostatic adenocarcinoma (monoclonal antibody against androgen receptors ×400)
DISCUSSION
In this study, it was found that all the patients of prostatic growth were in the older age group.
The mean age was 68.66 years. No significant age difference was detected between benign and malignant cases. Men et al. found similar age distribution. They found the mean age to be 64.67 years for prostatic growth lesions.[8]
NHP is the most common finding in routine histopathological examination followed by prostatic adenocarcinoma and PIN, respectively, the results corroborating the results of Men et al.
A majority of NHP cases had normal serum PSA values. Only three cases had the values falling in the grey zone. Two cases had significantly raised PSA values, whereas 50%-of PIN cases had PSA values falling in the grey zone. Among the 11 cases of carcinoma with raised PSA, 7 cases had it >100 ng/ml. Mean PSA was significantly higher in carcinoma than that of NHP and PIN. Serum PSA values were much higher in carcinoma than that of NHP with P value 0.00001, which is also corroborated by the results of study by Aboseif et al.[9]
In our study, 75%-of the adenocarcinoma cases showed higher Gleason scoring between 8 and 10. This finding is identical to the study results of Humprey.[10]
So, in our study, higher serum PSA was seen in cases with higher Gleason score showing a positive correlation.
ER expression in prostate tissues was negative in all cases of NHP, PIN and prostate carcinoma. These IHC results were identical to that of other studies such as Wernert[11] and Kang et al.[12] Wernert et al. found the ER and PR were demonstrated by IHC in nuclei of periglandular fibrocytes and smooth muscle cells and hyperplatic basal cells, but glandular secretory epithelium were negative and thus in prostatic carcinoma cases in their study ER and PR were negative. They concluded that estrogens might contribute to NHP by triggering stromal proliferation with a secondary inductive epithelial growth. Obviously, they do not act directly on prostatic carcinoma but inhibit growth via the hypophyseal-testicular axis. The biologic significance of the PR in prostatic carcinoma is unknown. The PR expressions of carcinoma cells and stromal cells in prostatic carcinoma were found in 93.3%-and 76.7%, respectively. The PR were immunoreactive in stromal cells around carcinoma cells, as demonstrated in studies done by Kang et al.,[12] Hiramatsu[13] and Bonkhoff et al.[14]
In our study, 82.35% of NHP cases showed PR [removed]including weak expression also), whereas 41.67% of carcinoma cases showed PR expression. In Fisher's exact test (two-tailed), a P value was 0.021 between NHP and carcinoma cases, which is statistically significant.
All the prostatic growth lesions in our study were positive for AR expression with varying staining intensity. Only 11.78% of NHP cases, 50% of PIN cases and 100% of carcinoma cases showed strongly positive AR expression. So, prostatic carcinoma cases showed highest content of AR among other prostatic lesions. It is statistically significant (P value < 0 xss=removed>et al.[15] and Brolin et al.[16] Qui-Yi-Q et al. had done a study to evaluate AR expression in clinically localized prostatic carcinoma. AR immunoreactivity is almost exclusively nuclear and was observed in tumor cells, non-neoplastic glandular epithelial cells and a proportion of peritumoral stromal cells. Mean percentages of AR-positive cells were significantly higher in cancer tissues compared to that in normal prostatic tissues (P < 0 xss=removed>et al. analyzed AR, PR and ER contents in cytosol and salt-extractable nuclear subcompartments from 6 normal, 39 NHP and 7 malignant prostatic tissue specimens using the radioligand-binding assay technique. The highest content was found in the cytosol and nucleic acid from malignant prostatic tissues.
CONCLUSION
From this study, we observed that among our patients with a mean age of 68.66 years whose specimens of prostate were sent from urology department to our pathology department for the histopathological study, NHP cases were most common and PIN were least common. Serum PSA was within normal limits in NHP cases, but higher in prostatic carcinoma cases and also more in higher Gleason score and grey-zone in PIN cases. Sex-steroid receptor status as seen by IHC was divergent in different types of prostatic growth lesions. PR was positive in most cases of NHP and almost half of carcinoma cases. All lesions showed positive staining of AR with highest expression in carcinoma cases. But, all cases were ER negative. With these findings, antiandrogen and antiprogesterone therapy may be indicated in the treatment of prostatic adenocarcinoma.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Jonathan I. Epstein- the lower urinary tract and male genital system, In: Kumar V, Abbas AK, Fausto N, Editors, Robbins and Cotran Pathologic basis of disease, 7 th ed, Chapter 21, Saunders: Elsevier; reprint 2006. p.1047,48,50,55.
- Yeole BB, Jussawalla DJ. Descriptive epidemiology of the cancers of male genital organs in greater Bombay. Indian J Cancer 1997;34:30-9.
- Rosai J, Ackerman. Male reproductive system, prostate and seminal vesicles. In: Rosai J, Ackerman, Editors. Rosai and Ackerman′s surgical pathology, 9 th ed, Vol. 1, Chapter 18, New Delhi: Mosby, Elsevier; reprint 2005. p. 1361-62,1369.
- Chakrabarti S, Raha K, Bhunia CL, Bhattachary DK. Department of Pathology, North Bengal Medical College and Hospital, Siliguri, The usefulness of prostate specific antigen density as a screening method for prostatic carcinoma. J Indian Med Assoc 2001;99:627-8, 630.
- Androgen receptor, Wikipedia, the free encyclopedia, 22 September, 2010.
- Bonkhoff H, Fixemer T, Hunsicker I, Remberger K. Estrogen receptor expression in prostate cancer and premalignant prostatic lesions. Am J Pathol 1999;155:641-7.
- Progesteron receptor, Wikipedia, the free encyclopedia, 18 September, 2010.
- Men S, Cakar B, Conkbayir I, Hekimoglu B. Detection of prostatic carcinoma: The role of TRUS, TRUS guided biopsy, digital rectal examination, PSA and PSA density. J Exp Clin Cancer Res 2001;20:473-80.
- Aboseif S, Shinohara K, Weidner N, Narayan P, Carroll PR. The significance of prostatic intra-epithelial neoplasia. Br J Urol 1995;76:355-9.
- Humphrey PA. Gleason grading and prognostic factors in carcinoma of the prostate. Mod Pathol 2004;17:292-306.
- ;Wernert N, Gerdes J, Loy V, Seitz G, Scherr O, Dhom G. Investigations of the estrogen (ER-ICA-test) and the progesterone receptor in the prostate and prostatic carcinoma on immunohistochemical basis. Virchows Arch A Pathol Anat Histopathol 1988:412:387-91.
- Kang MS, Park SY, Yoon HK. Estrogen and Progesterone Receptor Expressions in Benign Prostatic Hypertrophy and Prostatic Adenocarcinoma. Korean J Pathol 1998;32:346-51.
- Hiramatsu M, Maehara I, Orikasa S, Sasano H. Immunolocalization of oestrogen and progesterone receptors in prostatic hyperplasia and carcinoma. Histopathology 1996;28:163-8.
- Bonkhoff H, Fixemer T, Hunsicker I, Remberger K. Progesterone receptor expression in human prostate cancer: Correlation with tumor progression. Prostate 2001;48:285-91.
- Qiu YQ, Leuschner I, Braun PM. Androgen receptor expression in clinically localized prostate cancer: Immunohistochemistry study and literature review. Asian J Androl 2008;10:855-63.
- Brolin J, Andersson L, Ekman P. Steroid receptor profile and receptor stability in subfractions of human prostatic tissues, Urol Res 1991;19:327-31.

| Figure 1:(a) Histopathology of prostatic intraepithelial neoplasia (H/E ×400). (b) Histopathology of prostatic adenocarcinoma (H/E ×400)

| Figure 2:(a) Nodular hyperplasia of prostate (monoclonal antibody against progesterone receptors ×1000). (b) Prostatic adenocarcinoma (monoclonal antibody against progesterone receptors ×400)
References
- Jonathan I. Epstein- the lower urinary tract and male genital system, In: Kumar V, Abbas AK, Fausto N, Editors, Robbins and Cotran Pathologic basis of disease, 7 th ed, Chapter 21, Saunders: Elsevier; reprint 2006. p.1047,48,50,55.
- Yeole BB, Jussawalla DJ. Descriptive epidemiology of the cancers of male genital organs in greater Bombay. Indian J Cancer 1997;34:30-9.
- Rosai J, Ackerman. Male reproductive system, prostate and seminal vesicles. In: Rosai J, Ackerman, Editors. Rosai and Ackerman′s surgical pathology, 9 th ed, Vol. 1, Chapter 18, New Delhi: Mosby, Elsevier; reprint 2005. p. 1361-62,1369.
- Chakrabarti S, Raha K, Bhunia CL, Bhattachary DK. Department of Pathology, North Bengal Medical College and Hospital, Siliguri, The usefulness of prostate specific antigen density as a screening method for prostatic carcinoma. J Indian Med Assoc 2001;99:627-8, 630.
- Androgen receptor, Wikipedia, the free encyclopedia, 22 September, 2010.
- Bonkhoff H, Fixemer T, Hunsicker I, Remberger K. Estrogen receptor expression in prostate cancer and premalignant prostatic lesions. Am J Pathol 1999;155:641-7.
- Progesteron receptor, Wikipedia, the free encyclopedia, 18 September, 2010.
- Men S, Cakar B, Conkbayir I, Hekimoglu B. Detection of prostatic carcinoma: The role of TRUS, TRUS guided biopsy, digital rectal examination, PSA and PSA density. J Exp Clin Cancer Res 2001;20:473-80.
- Aboseif S, Shinohara K, Weidner N, Narayan P, Carroll PR. The significance of prostatic intra-epithelial neoplasia. Br J Urol 1995;76:355-9.
- Humphrey PA. Gleason grading and prognostic factors in carcinoma of the prostate. Mod Pathol 2004;17:292-306.
- ;Wernert N, Gerdes J, Loy V, Seitz G, Scherr O, Dhom G. Investigations of the estrogen (ER-ICA-test) and the progesterone receptor in the prostate and prostatic carcinoma on immunohistochemical basis. Virchows Arch A Pathol Anat Histopathol 1988:412:387-91.
- Kang MS, Park SY, Yoon HK. Estrogen and Progesterone Receptor Expressions in Benign Prostatic Hypertrophy and Prostatic Adenocarcinoma. Korean J Pathol 1998;32:346-51.
- Hiramatsu M, Maehara I, Orikasa S, Sasano H. Immunolocalization of oestrogen and progesterone receptors in prostatic hyperplasia and carcinoma. Histopathology 1996;28:163-8.
- Bonkhoff H, Fixemer T, Hunsicker I, Remberger K. Progesterone receptor expression in human prostate cancer: Correlation with tumor progression. Prostate 2001;48:285-91.
- Qiu YQ, Leuschner I, Braun PM. Androgen receptor expression in clinically localized prostate cancer: Immunohistochemistry study and literature review. Asian J Androl 2008;10:855-63.
- Brolin J, Andersson L, Ekman P. Steroid receptor profile and receptor stability in subfractions of human prostatic tissues, Urol Res 1991;19:327-31.


 PDF
PDF  Views
Views  Share
Share

