Acute Lymphoblastic Leukemia as Secondary Malignancy in a Case of Ewing’s Sarcoma on Treatment
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(03): 354-356
DOI: DOI: 10.4103/ijmpo.ijmpo_110_16
Abstract
The survival of Ewing's sarcoma (ES) has improved due to advances in both local and systemic therapy. This has given rise to an increased detection of second malignant neoplasms which can be in the form of solid tumors and hematological malignancies. The most common hematological malignancies are acute myeloid leukemia/myelodysplastic syndrome. Acute lymphoblastic leukemia (ALL) is relatively uncommon in occurrence in this setting. Furthermore, the average refractory period for hematological malignancies varies from 3 to 5 years. We report a case of a young female who developed ALL while on adjuvant therapy for ES.
Publication History
Article published online:
04 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
The survival of Ewing's sarcoma (ES) has improved due to advances in both local and systemic therapy. This has given rise to an increased detection of second malignant neoplasms which can be in the form of solid tumors and hematological malignancies. The most common hematological malignancies are acute myeloid leukemia/myelodysplastic syndrome. Acute lymphoblastic leukemia (ALL) is relatively uncommon in occurrence in this setting. Furthermore, the average refractory period for hematological malignancies varies from 3 to 5 years. We report a case of a young female who developed ALL while on adjuvant therapy for ES.
Introduction
The 5-year survival rate for Ewing's sarcoma (ES)/ES family of tumors has increased from 20%-before the use of systemic therapy in the early 1970s[1] to around 70%-with the current therapy in localized disease.[2] This improvement in survival is credited to improved surgical techniques, refinements in radiation therapy (RT), and intensified multiagent chemotherapy. In ES as well as in all childhood cancers, late effects are being discovered as survival rates improve and the survivor pool enlarges. Secondary malignant neoplasms (SMNs) have been recognized as a particularly tragic late effect of childhood cancer therapy. RT, anthracyclines, alkylators, and epipodophyllotoxins are all used in modern ES therapy and have been associated with SMN.
The overall incidence of SMN in ES is about 6%–8%-in various studies.[3] In one study, the risk of developing an SMN at 5, 10, and 20 years was 2.1%, 4.4%, and 8%, respectively.[4]
The most common solid tumor SMN after ES is osteosarcoma (OS), followed by malignant fibrous histiocytoma.[5,6,7,8,9] OS has been noted to comprise about 50%–60%[5,9,10,11] of solid tumor SMN although OS has made up a smaller percentage after therapy in more recent eras.[12]
The most common hematologic SMN in all reports is acute myeloid leukemia (AML)/myelodysplastic syndrome (MDS), which comprises about 60%-of cases.[3,4,13] Other hematologic SMN includes both B-cell and T-cell lineage acute lymphoblastic leukemia (ALL),[5,13,14,15] Hodgkin's lymphoma,[4,10] non-Hodgkin's lymphoma,[4,16] and multiple myeloma.[4]
Hematologic SMN occurring after a shorter latency from diagnosis (36 months vs. 98 months for solid tumors) was noted in many of the above reports.
No known hereditary cancer syndromes have ever been associated with ES (although ES occurring as SMN after treatment for heritable RB continues to be described in the literature).[17,18,19,20,21,22] Furthermore, there have been no reports of development of ALL as a complication of RT.
Although development of therapy-related MDS and AML are well-known occurrences with the use of alkylating agents and epipodophyllotoxins, a secondary ALL is rarely encountered in clinical practice.
We report a case of a young female who developed ALL, while on adjuvant treatment for ES.
Case Report
A 15-year-old female presented with pain and swelling in the left knee joint for 1 month duration. There were no other complaints and no significant history was present. There was no family history of any cancer. She had no addictions and her menstrual history was normal.
On examination, it was a 6 cm × 6 cm swelling which was mildly tender without any signs of inflammation. Systemic examination was unremarkable.
Magnetic resonance imaging of the left knee joint showed the lesion in distal metaphysis of left femur with permeative destruction and perilesional edema. A bone scan revealed no other site of increased radiotracer concentration. A normal computed tomography scan of the thorax proved the disease to be nonmetastatic.
Histopathologically, the lesion was shown to be ES which was confirmed by immunohistochemistry.
The patient was started on neoadjuvant chemotherapy-ifosfamide, vincristine, adriamycin, and actinomycin D protocol.[23] The treatment was complicated by development of generalized tonic–clonic seizures during administration of second cycle. Central nervous system imaging revealed an infarct in left frontal lobe, the etiology of which could not be specified. Subsequently, she was given the third cycle with antiepileptics which was complicated by hemorrhagic cystitis.
The patient was then started on a different vincristine, actinomycin D, cyclophosphamide, and adriamycin (VACA) protocol which is devoid if ifosfamide in view of poor tolerance. One cycle of VACA protocol was given followed by limb salvage surgery (wide local excision and total knee replacement). On histopathological examination of the surgical specimen, there was 30%–35%-necrosis and 40%–50%-viable tumor. This was followed by curative radiotherapy to tumor site at a dose of 54 Gy in 27 fractions with concurrent administration of weekly vincristine and cyclophosphamide.
The patient was then resumed on VACA protocol as adjuvant chemotherapy. During second cycle of adjuvant chemotherapy, her complete blood count revealed persistent leukocytosis and thrombocytopenia. Manual differential count revealed 77%-of immature blast cells on peripheral smear [Figure 1]. Bone marrow examination revealed hypercellular marrow with 86%-blasts which were morphologically lymphoid [Figure 2]. On immunophenotyping, blasts were positive for CD 19 and CD 34 and negative for CD 13 making the final diagnosis to be pre-B-cell-ALL.
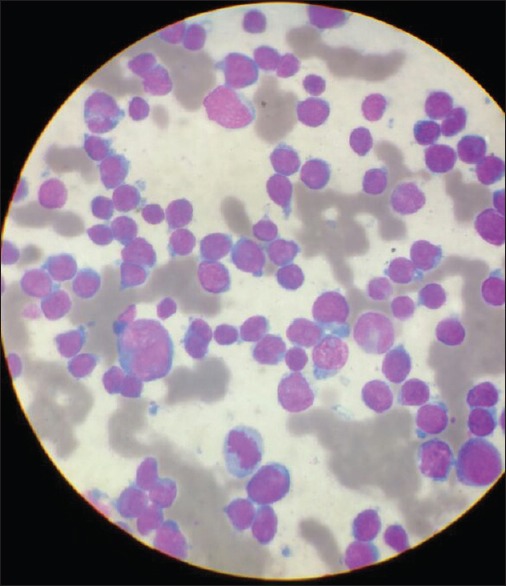
| Figure 1:Peripheral smear showing presence of blast cells

| Figure 2:Bone marrow examination showing excess of lymphoblasts
Conventional karyotyping was normal. Bcr-abl chromosome by fluorescence in situ hybridization was not detected. Cerebrospinal fluid cytology was negative for malignant cells.
Thus, she developed ALL while on treatment for ES (after a time period of around 1 year of starting the treatment).
Discussion
Although cases of secondary ALL with the use of alkylators and epipodophyllotoxins have been reported,[5,13,14,15] it is much more likely to encounter myeloid leukemia/MDS as second malignancies with the use of these agents. In particular, cyclophosphamide has been linked to monosomy 5 and monosomy 7 associated MDS with a latency of 5 years or more, and etoposide has been linked to 11q23 associated AML with a latency of 2 years or less.[24,25,26] The hematological SMN after ES has also predominantly been AML/MDS.[3,4,13]
The above-mentioned patient had not received etoposide anytime during her treatment for ES. She was given ifosfamide at a cumulative dose of 27 g/m2 and subsequently cyclophosphamide at a cumulative dose of 8.4 g/m2. The cumulative dose of doxorubicin given was 300 mg/m2.
The above-mentioned case was remarkable in two aspects. First, development of ALL as compared to myeloid malignancies is a rare event as far as secondary malignancies are concerned. Second, secondary malignancies after alkylating agents typically occur after a latent period of 5–6 years; however, in this particular patient, it developed in just over 1 year from the primary diagnosis.
Conclusion
As the survival of patients of ES increases with involvement of multimodality therapy, there remains a small yet significant concern of the unfortunate occurrence of a secondary malignancy in these otherwise cured patients. Thus, the clinician should keep in mind the possibility of the same while following up such patients and even, as seen in the above case, while administering adjuvant therapy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Phillips RF, Higinbotham NL. The curability of Ewing's endothelioma of bone in children. J Pediatr 1967;70:391-7.
- Grier HE, Krailo MD, Tarbell NJ, Link MP, Fryer CJ, Pritchard DJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med 2003;348:694-701.
- Burdach S, van Kaick B, Laws HJ, Ahrens S, Haase R, Körholz D, et al. Allogeneic and autologous stem-cell transplantation in advanced Ewing tumors. An update after long-term follow-up from two centers of the European Intergroup study EICESS. Stem-Cell Transplant Programs at Düsseldorf University Medical Center, Germany and St. Anna Kinderspital, Vienna, Austria. Ann Oncol 2000;11:1451-62.
- Sultan I, Rihani R, Hazin R, Rodriguez-Galindo C. Second malignancies in patients with Ewing sarcoma family of tumors: A population-based study. Acta Oncol 2010;49:237-44.
- Kuttesch JF Jr., Wexler LH, Marcus RB, Fairclough D, Weaver-McClure L, White M, et al. Second malignancies after Ewing's sarcoma: Radiation dose-dependency of secondary sarcomas. J Clin Oncol 1996;14:2818-25.
- Bacci G, Longhi A, Barbieri E, Ferrari S, Mercuri M, Briccoli A, et al. Second malignancy in 597 patients with Ewing sarcoma of bone treated at a single institution with adjuvant and neoadjuvant chemotherapy between 1972 and 1999. J Pediatr Hematol Oncol 2005;27:517-20.
- Fuchs B, Valenzuela RG, Petersen IA, Arndt CA, Sim FH. Ewing's sarcoma and the development of secondary malignancies. Clin Orthop Relat Res 2003;(415):82-9.
- Wagner LM, Neel MD, Pappo AS, Merchant TE, Poquette CA, Rao BN, et al. Fractures in pediatric Ewing sarcoma. J Pediatr Hematol Oncol 2001;23:568-71.
- Paulino AC, Fowler BZ. Secondary neoplasms after radiotherapy for a childhood solid tumor. Pediatr Hematol Oncol 2005;22:89-101.
- McLean TW, Hertel C, Young ML, Marcus K, Schizer MA, Gebhardt M, et al. Late events in pediatric patients with Ewing sarcoma/primitive neuroectodermal tumor of bone: The Dana-Farber Cancer Institute/Children's Hospital experience. J Pediatr Hematol Oncol 1999;21:486-93.
- Hawkins MM, Wilson LM, Burton HS, Potok MH, Winter DL, Marsden HB, et al. Radiotherapy, alkylating agents, and risk of bone cancer after childhood cancer. J Natl Cancer Inst 1996;88:270-8.
- Goldsby R, Burke C, Nagarajan R, Zhou T, Chen Z, Marina N, et al. Second solid malignancies among children, adolescents, and young adults diagnosed with malignant bone tumors after 1976: Follow-up of a Children's Oncology Group cohort. Cancer 2008;113:2597-604.
- Bhatia S, Krailo MD, Chen Z, Burden L, Askin FB, Dickman PS, et al. Therapy-related myelodysplasia and acute myeloid leukemia after Ewing sarcoma and primitive neuroectodermal tumor of bone: A report from the Children's Oncology Group. Blood 2007;109:46-51.
- Bacci G, Toni A, Avella M, Manfrini M, Sudanese A, Ciaroni D, et al. Long-term results in 144 localized Ewing's sarcoma patients treated with combined therapy. Cancer 1989;63:1477-86.
- Paulussen M, Ahrens S, Lehnert M, Taeger D, Hense HW, Wagner A, et al. Second malignancies after Ewing tumor treatment in 690 patients from a cooperative German/Austrian/Dutch study. Ann Oncol 2001;12:1619-30.
- Rossbach HC, Chamizo W, Walling AK, Grana NH, Washington K, Barbosa JL. Ki-1+large-cell anaplastic lymphoma after Ewing sarcoma. J Pediatr Hematol Oncol 1999;21:50-2.
- Cope JU, Tsokos M, Miller RW. Ewing sarcoma and sinonasal neuroectodermal tumors as second malignant tumors after retinoblastoma and other neoplasms. Med Pediatr Oncol 2001;36:290-4.
- Spunt SL, Rodriguez-Galindo C, Fuller CE, Harper J, Krasin MJ, Billups CA, et al. Ewing sarcoma-family tumors that arise after treatment of primary childhood cancer. Cancer 2006;107:201-6.
- ;Ceha HM, Balm AJ, de Jong D, van 't Veer LJ. Multiple malignancies in a patient with bilateral retinoblastoma. J Laryngol Otol 1998;112:189-92.
- Helton KJ, Fletcher BD, Kun LE, Jenkins JJ 3rd, Pratt CB. Bone tumors other than osteosarcoma after retinoblastoma. Cancer 1993;71:2847-53.
- Mittal R, Al Awadi S, Sahar O, Behbehani AM. Ewing's sarcoma as second malignant neoplasm after retinoblastoma: A case report. Med Princ Pract 2008;17:84-5.
- Mohney BG, Robertson DM, Schomberg PJ, Hodge DO. Second nonocular tumors in survivors of heritable retinoblastoma and prior radiation therapy. Am J Ophthalmol 1998;126:269-77.
- Craft A, Cotterill S, Malcolm A, Spooner D, Grimer R, Souhami R, et al. Ifosfamide-containing chemotherapy in Ewing's sarcoma: The Second United Kingdom Children's Cancer Study Group and the Medical Research Council Ewing's Tumor Study. J Clin Oncol 1998;16:3628-33.
- Pedersen-Bjergaard J, Pedersen M, Roulston D, Philip P. Different genetic pathways in leukemogenesis for patients presenting with therapy-related myelodysplasia and therapy-related acute myeloid leukemia. Blood 1995;86:3542-52.
- Super HJ, McCabe NR, Thirman MJ, Larson RA, Le Beau MM, Pedersen-Bjergaard J, et al. Rearrangements of the MLL gene in therapy-related acute myeloid leukemia in patients previously treated with agents targeting DNA-topoisomerase II. Blood 1993;82:3705-11.
- Thirman MJ, Gill HJ, Burnett RC, Mbangkollo D, McCabe NR, Kobayashi H, et al. Rearrangement of the MLL gene in acute lymphoblastic and acute myeloid leukemias with 11q23 chromosomal translocations. N Engl J Med 1993;329:909-14.

| Figure 1:Peripheral smear showing presence of blast cells

| Figure 2:Bone marrow examination showing excess of lymphoblasts
References
- Phillips RF, Higinbotham NL. The curability of Ewing's endothelioma of bone in children. J Pediatr 1967;70:391-7.
- Grier HE, Krailo MD, Tarbell NJ, Link MP, Fryer CJ, Pritchard DJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med 2003;348:694-701.
- Burdach S, van Kaick B, Laws HJ, Ahrens S, Haase R, Körholz D, et al. Allogeneic and autologous stem-cell transplantation in advanced Ewing tumors. An update after long-term follow-up from two centers of the European Intergroup study EICESS. Stem-Cell Transplant Programs at Düsseldorf University Medical Center, Germany and St. Anna Kinderspital, Vienna, Austria. Ann Oncol 2000;11:1451-62.
- Sultan I, Rihani R, Hazin R, Rodriguez-Galindo C. Second malignancies in patients with Ewing sarcoma family of tumors: A population-based study. Acta Oncol 2010;49:237-44.
- Kuttesch JF Jr., Wexler LH, Marcus RB, Fairclough D, Weaver-McClure L, White M, et al. Second malignancies after Ewing's sarcoma: Radiation dose-dependency of secondary sarcomas. J Clin Oncol 1996;14:2818-25.
- Bacci G, Longhi A, Barbieri E, Ferrari S, Mercuri M, Briccoli A, et al. Second malignancy in 597 patients with Ewing sarcoma of bone treated at a single institution with adjuvant and neoadjuvant chemotherapy between 1972 and 1999. J Pediatr Hematol Oncol 2005;27:517-20.
- Fuchs B, Valenzuela RG, Petersen IA, Arndt CA, Sim FH. Ewing's sarcoma and the development of secondary malignancies. Clin Orthop Relat Res 2003;(415):82-9.
- Wagner LM, Neel MD, Pappo AS, Merchant TE, Poquette CA, Rao BN, et al. Fractures in pediatric Ewing sarcoma. J Pediatr Hematol Oncol 2001;23:568-71.
- Paulino AC, Fowler BZ. Secondary neoplasms after radiotherapy for a childhood solid tumor. Pediatr Hematol Oncol 2005;22:89-101.
- McLean TW, Hertel C, Young ML, Marcus K, Schizer MA, Gebhardt M, et al. Late events in pediatric patients with Ewing sarcoma/primitive neuroectodermal tumor of bone: The Dana-Farber Cancer Institute/Children's Hospital experience. J Pediatr Hematol Oncol 1999;21:486-93.
- Hawkins MM, Wilson LM, Burton HS, Potok MH, Winter DL, Marsden HB, et al. Radiotherapy, alkylating agents, and risk of bone cancer after childhood cancer. J Natl Cancer Inst 1996;88:270-8.
- Goldsby R, Burke C, Nagarajan R, Zhou T, Chen Z, Marina N, et al. Second solid malignancies among children, adolescents, and young adults diagnosed with malignant bone tumors after 1976: Follow-up of a Children's Oncology Group cohort. Cancer 2008;113:2597-604.
- Bhatia S, Krailo MD, Chen Z, Burden L, Askin FB, Dickman PS, et al. Therapy-related myelodysplasia and acute myeloid leukemia after Ewing sarcoma and primitive neuroectodermal tumor of bone: A report from the Children's Oncology Group. Blood 2007;109:46-51.
- Bacci G, Toni A, Avella M, Manfrini M, Sudanese A, Ciaroni D, et al. Long-term results in 144 localized Ewing's sarcoma patients treated with combined therapy. Cancer 1989;63:1477-86.
- Paulussen M, Ahrens S, Lehnert M, Taeger D, Hense HW, Wagner A, et al. Second malignancies after Ewing tumor treatment in 690 patients from a cooperative German/Austrian/Dutch study. Ann Oncol 2001;12:1619-30.
- Rossbach HC, Chamizo W, Walling AK, Grana NH, Washington K, Barbosa JL. Ki-1+large-cell anaplastic lymphoma after Ewing sarcoma. J Pediatr Hematol Oncol 1999;21:50-2.
- Cope JU, Tsokos M, Miller RW. Ewing sarcoma and sinonasal neuroectodermal tumors as second malignant tumors after retinoblastoma and other neoplasms. Med Pediatr Oncol 2001;36:290-4.
- Spunt SL, Rodriguez-Galindo C, Fuller CE, Harper J, Krasin MJ, Billups CA, et al. Ewing sarcoma-family tumors that arise after treatment of primary childhood cancer. Cancer 2006;107:201-6.
- ;Ceha HM, Balm AJ, de Jong D, van 't Veer LJ. Multiple malignancies in a patient with bilateral retinoblastoma. J Laryngol Otol 1998;112:189-92.
- Helton KJ, Fletcher BD, Kun LE, Jenkins JJ 3rd, Pratt CB. Bone tumors other than osteosarcoma after retinoblastoma. Cancer 1993;71:2847-53.
- Mittal R, Al Awadi S, Sahar O, Behbehani AM. Ewing's sarcoma as second malignant neoplasm after retinoblastoma: A case report. Med Princ Pract 2008;17:84-5.
- Mohney BG, Robertson DM, Schomberg PJ, Hodge DO. Second nonocular tumors in survivors of heritable retinoblastoma and prior radiation therapy. Am J Ophthalmol 1998;126:269-77.
- Craft A, Cotterill S, Malcolm A, Spooner D, Grimer R, Souhami R, et al. Ifosfamide-containing chemotherapy in Ewing's sarcoma: The Second United Kingdom Children's Cancer Study Group and the Medical Research Council Ewing's Tumor Study. J Clin Oncol 1998;16:3628-33.
- Pedersen-Bjergaard J, Pedersen M, Roulston D, Philip P. Different genetic pathways in leukemogenesis for patients presenting with therapy-related myelodysplasia and therapy-related acute myeloid leukemia. Blood 1995;86:3542-52.
- Super HJ, McCabe NR, Thirman MJ, Larson RA, Le Beau MM, Pedersen-Bjergaard J, et al. Rearrangements of the MLL gene in therapy-related acute myeloid leukemia in patients previously treated with agents targeting DNA-topoisomerase II. Blood 1993;82:3705-11.
- Thirman MJ, Gill HJ, Burnett RC, Mbangkollo D, McCabe NR, Kobayashi H, et al. Rearrangement of the MLL gene in acute lymphoblastic and acute myeloid leukemias with 11q23 chromosomal translocations. N Engl J Med 1993;329:909-14.


 PDF
PDF  Views
Views  Share
Share

