Advanced Thyroid Cancer Controversy and Consensus
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2020; 41(04): 476-480
DOI: DOI: 10.4103/ijmpo.ijmpo_145_20
The overall incidence of differentiated thyroid cancers displaying aggressive behavior, by local invasion, distant metastasis, treatment resistance, and increased mortality ranges between 10%-and 15%.[1],[2],[3],[4] Due to this relatively low incidence and unstandardized definition for locally advanced thyroid cancers (LATC), there is a great amount of discrepancy regarding the definition and management of LATC. There is also a lack of good quality prospective studies to guide the correct approach and management of this group of patients. Majority of existing papers have a distinct heterogeneity in the subject population and disease characteristics. All existing guidelines are derived from retrospective observational studies with possible bias and incomplete data.
In this review, we would like to highlight the main controversies and possible consensus in the management of LATCs.
Publication History
Received: 09 April 2020
Accepted: 11 June 2020
Article published online:
17 May 2021
© 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
The overall incidence of differentiated thyroid cancers displaying aggressive behavior, by local invasion, distant metastasis, treatment resistance, and increased mortality ranges between 10%-and 15%.[1],[2],[3],[4] Due to this relatively low incidence and unstandardized definition for locally advanced thyroid cancers (LATC), there is a great amount of discrepancy regarding the definition and management of LATC. There is also a lack of good quality prospective studies to guide the correct approach and management of this group of patients. Majority of existing papers have a distinct heterogeneity in the subject population and disease characteristics. All existing guidelines are derived from retrospective observational studies with possible bias and incomplete data.
In this review, we would like to highlight the main controversies and possible consensus in the management of LATCs.
Controversies in Locally Advanced Thyroid Cancers
Definition of locally advanced thyroid cancers
There is a lack of consensus in defining locally advanced thyroid cancers. The most commonly accepted definition of LATC is: any thyroid cancer, with tumor or nodal disease involving the recurrent laryngeal nerves (RLN), aerodigestive tract, great vessels (internal jugular vein and internal carotid artery), extensive soft-tissue involvement with or without multiple bulky bilateral nodal metastasis to the central or lateral compartment.
Preoperative evaluation
A sound clinical acumen is indispensable for the preoperative recognition of LATC. There are specific signs and symptoms associated with the disease invading into the surrounding structures, which must be recognized to raise suspicion of it being a LATC. Furthermore, it is important to note that the majority of LATCs maybe asymptomatic. Hence, in many cases, clinical examination and symptomatology maybe the only guide to investigate further for LATC and to plan the necessary treatment strategies.
The common symptoms which will guide the clinician to suspect LATC include change of voice, voice weakness, vocal fatigue or hoarseness, suggesting RLN or laryngeal involvement; dyspnoea, stridor, hemoptysis, suggesting laryngotracheal framework involvement; and odynophagia and/or dysphagia, suggesting pharyngeal or esophageal involvement. Neck pain, neck stiffness, and inability to extend the neck suggest involvement of the prevertebral muscle or fascia. The presence of large cervical metastatic nodes and/or inability to feel the lower border of the thyroid swelling may suggest mediastinal extension.
Routine ultrasonography alone may not be sufficient to map the extent of the disease in LATC, and cross-sectional imaging is required for the complete evaluation of a thyroid nodule with clinical signs and symptoms of extrathyroid extension. Contrast-enhanced computed tomography (CECT) is the diagnostic imaging of choice in most patients where one suspects advanced disease.[5] The sensitivity and specificity of predicting RLN involvement by CECT were found to be 78.2%- and 89.8%, respectively. Tracheal infiltration can be assessed by the degree of tumor contact with the trachea [Figure 1], a contact >90°of tracheal circumference on a computed tomography (CT) scan is sensitive in 68.1%-and specific in 76.6%-for the prediction of tracheal involvement.[6] Shin et al. [7] described the pathological staging of papillary thyroid carcinoma with airway invasion. In their article, a system of five stages of tracheal invasion based on the resected specimens was described. There is, however, no published data on the validation of CECT for Shin's pathological staging of tracheal invasion, but radiologists should be encouraged to report tracheal involvement by thyroid cancers as per Shin's classification. With regard to esophageal involvement, CT scan may not have a good low sensitivity to detect invasion into the layers of the esophageal wall, especially the outer layer.[6]
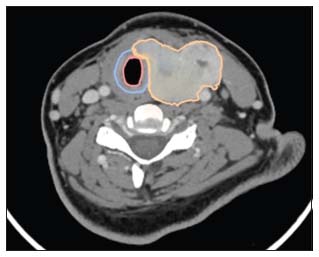
| Figure 1:Contrast enhanced computed tomography scan of locally advanced thyroid cancer with disease infiltrating into the trachea without mucosal breach (Shin III). (Orange – tumour outline, Blue – Tracheal cartilage and Pink – Tracheal mucosa)
Magnetic resonance imaging (MRI) may add to CECT for the prediction of invasion into the RLN, laryngotracheal complex, and esophagus. Although MRI has a slight edge over CECT to predict the invasion into the esophagus and RLN, it lacks the ability to predict the depth of tracheal invasion in comparison with CECT.[6],[8],[9] Esophageal involvement is a rare occurrence even in LATCs, and MRI is generally reserved as a problem-solving tool.
Tracheo-bronchoscopy and esophagoscopy may provide additional information about the invasiveness of the disease and help in surgical planning. Endoscopic ultrasonography (EUS) has a better accuracy than the conventional esophagoscopy and MRI to detect in the esophageal infiltration.[10],[11] The EUS was able to better detect the depth of infiltration into the esophagus leading to accurate surgical plan.[10] EUS has its drawbacks because (a) the upper portions of the thyroid gland cannot be imaged by EUS, primarily due to the lack of all five esophageal layers at this site, as the lower pharyngeal constrictor muscle transforms to the esophageal muscularis propria and the gag reflex and (b) EUS also cannot determine minor muscular propria invasion.[10],[11]
Surgical Issues
An attempt to perform a complete resection (R0) is vital and offers the patient the best possible chance of cure.[12] As per the Memorial Sloan Kettering LATC case series, elderly patients, tumors more than 4 cm in size, gross residual disease, and distant metastases were the predictors of poor survival.[12] The spectrum of surgeries performed in patients with LATC range from a total thyroidectomy with bilateral central and lateral neck dissections to multiorgan resections, sometimes requiring sternotomies and vascular resections. The aim of performing such extensive surgeries is to achieve R0/R1 resections, thus optimizing the outcomes. A balance between the morbidity of such surgeries, the poor prognostic factors, and the possible outcomes should be maintained before decision-making. Radical surgeries must not be performed in poor surgical candidates and in patients with poor prognosis.
Intraoperative neuromonitoring use versus no use
Intraoperative neuromonitoring (IONM) can help the surgeon identify the RLN in a distorted anatomy, it can estimate the residual nerve function following surgery, it can provide pointers to decision-making when both the RLN are at risk due to bilateral disease and the postoperative residual nerve potential might provide clues to possible postoperative complications. The exact role of IONM has not been clearly defined and whether IONM can decrease the incidence of temporary or permanent RLN palsy, especially in these cancers, is a matter of contention.
In a metanalysis by Bai and Chen consisting of 34 studies, they found a significant decrease in total injury, transient injury, and permanent injury with IONM. A subgroup analysis reported that IONM had a prevented the complete, permanent, and transient RLN paralysis in total thyroidectomies. It also reduced the rate of complete and transient RLN injury in thyroidectomies for malignancies.[13] However, a Cochrane systematic review by Roberto Cirocchi in 2019 showed that there was no distinct advantage of IONM versus visual identification in the permanent and transient RLN palsies.
It is also found that IONM is cost-effective when the prevalence of transient nerve injury is >38%-and in high-volume centers with >5 IONM procedures per week.[14],[15]
IONM may not be mandatory for every thyroidectomy, but, surgeons should use IONM, if they feel more comfortable using the device during thyroidectomy, especially for LATCs where there is a chance of distorted anatomy. In LATC, where bilateral RLNs are engulfed and/or involved, IONM may guide the surgeon to assess the functional integrity of the RLNs following a complete dissection, in addition to its anatomical integrity. Vagal stimulation before and after dissection helps to confirm the critical point of injury and may guide us in identifying the correct segment in case of a segmental injury. The advances in continuous IONM technology may minimize the limitations of intermittent IONM monitoring.[16] However, IONM should not replace anatomical identification of the nerve and following of correct techniques of surgery
Recurrent laryngeal nerve (save vs. sacrifice)
Management of recurrent laryngeal nerve during surgery for LATCs is critical, Falk and McCaffrey and Nishida et al. studied the effect of RLN preservation versus resection in functionally intact nerves in LATC cases. They found that there were no differences in survival when the RLN was preserved, if patients received adjuvant RAI therapy with TSH suppression and there was no gross residual following resection.[4],[17] The basic principle of management of the RLN during surgery for LATCs is to make all attempts to dissect the nerve out of the tumor/nodal mass and preserve the continuity, without leaving back gross residual disease.
A nonfunctioning RLN involved by tumor may be resected en bloc with the thyroid resection. However, if there is no evidence of tumor infiltrating the RLN, then even in a nonfunctional nerve, it must be meticulously dissected and preserved to allow for recovery.[17],[18] The use of IONM here may help in determining the amplitude of EMG, suggesting the possible recovery pattern in the postoperative setting.
If the RLN is resected for oncological purposes, every attempt should be made to reconstruct the nerve using the ansa cervicalis or other nerve grafts. This would only be possible if the proximal and distal nerve ends are available for anastomosis. Typically, this is not feasible when the disease is infiltrating the nerve at the entry point, near the Berry's ligament. This kind of resection and anastomosis is generally possible when a nodal mass, rather than a primary thyroid tumor, involves the nerve.
[Table 1] summarizes the factors determining the RLN sacrifice v/s safety as per the International neuromonitoring study group guidelines 2018.[19]
|
Favouring nerve sacrifice |
Favouring nerve sparing |
|---|---|
|
RLN – Recurrent laryngeal nerves; EBRT – External-beam radiotherapy; RAI – Radioactive iodine |
|
|
Aggressive histology and genetic variants |
Young patients, generally radioiodine avid patients, papillary thyroid carcinoma |
|
Iodine refractory, previous EBRT, recurrence following adjuvant therapy |
Good efficacy to RAI and EBRT |
|
Nerve invasion at point of entry to the larynx |
Elderly (increased risk of aspiration) |
|
Normal contralateral vocal cord function |
Reduced pulmonary capacity |
|
No distant metastasis |
Voice professionals |
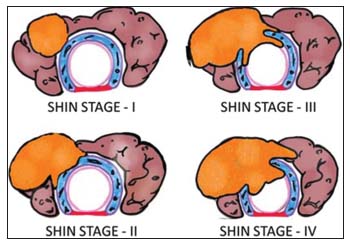
| Figure.2:Shin staging: Thyroid cancers invading the trachea: (top left) Stage I, (bottom left) Stage II, (top right) Stage III, and (bottom right) Stage IV (diagram conceptualized from Shin et al. [7])
Stage I: invasion of the capsule of the thyroid with the disease abutting the outer perichondrium of the trachea without eroding the cartilage. Stage II: Outer tracheal cartilage invasion, either in between the tracheal rings or destroying the cartilage. Stage III: tracheal cartilage invasion or extension between the rings into the underlying tracheal lamina propria without mucosal invasion. Stage IV: a complete tracheal cartilage invasion with intraluminal disease, seen as ulceration.[7]
Intraoperatively, tracheal shaving of the tumor can be done for Shin I tumors, a window/sleeve resection can be advocated in Shin II tumors, with lateral or anterior invasion of trachea. Circumferential resection is generally performed in patients with Shin III and IV tumors.[20] All attempts should be made to avoid leaving back gross residual disease on the trachea as this is associated with poorer outcomes.[21],[22],[23]
Esophageal involvement
This invasion typically occurs along with tracheal invasion but can also occur when paratracheal or paraesophageal lymph nodes have extranodal extension. It is usually considered a poor prognostic factor and correlates with significant reduction in overall survival.[17],[20] All attempts should be made to perform a simple cuff excision of the muscularis layer of the esophagus, and in most cases, this is adequate. Segmental resection should be performed if the tumor is extensively involving all three layers. Appropriate reconstruction will be required if full-thickness excisions are needed and a preoperative suspicion of esophageal involvement will help plan this better.
Adjuvant therapy
The evidence for use of adjuvant external-beam radiation therapy (EBRT) in advanced thyroid cancers is derived from retrospective studies, and it has been found to offer a small benefit in improving the locoregional control and in R1 resections with gross Educational Testing Service and multiple nodes with perinodal extension.[24] In a systematic review by Fussey et al., it was found that the use of EBRT in patients with thyroid cancer, improved the locoregional control in the high risk cases and those aged over 45 years.[25] A case series from the Memorial Sloan-Kettering institute showed that EBRT was effective in advanced and recurrent thyroid cancers with acceptable acute toxicities.[26] Intensity-modulated radiotherapy has shown to have limited toxicity in these patients and should be the radiation technique if choice. However, there have been two large retrospective studies which did not show improvement in the outcomes with the use of EBRT.[27],[28]
Therefore, there is no consensus on the routine use of EBRT in patients with LATC with R0/R1 resection and RAI therapy may suffice in them. In the absence of any prospective data on this issue, decisions for adjuvant RT should be made after a multidisciplinary discussion along with the radiation oncologist, endocrinologist, pathologist, and nuclear medicine physicians. It is important to balance the risks with benefits before decision of use of EBRT in patients with complete resections and well-differentiated thyroid cancers.
Conclusion
Management of locally advanced thyroid cancer remains a contentious subject. Much of the disagreements are centred around workup, extent of thyroidectomy, neuromonitoring, and adjuvant therapy. Precise patient assessment, comprehensive clinical and radiological evaluation is vital for therapeutic planning. A cross-sectional imaging in the form of CECT or MRI will aid in the assessment of extent of the disease and status of surrounding visceral and neural structures. Aggressive surgical en bloc resection of all gross disease with and possibly preservation of critical structures, when oncologically safe, along with adjuvant therapy (RAI with or without EBRT) provides the best outcomes.
References
- Britto E, Shah S, Parikh DM, Rao RS. Laryngotracheal invasion by well-differentiated thyroid cancer: Diagnosis and management. J Surg Oncol 1990; 44: 25-31
- Djalilian M, Beahrs OH, Devine KD, Weiland LH, DeSanto LW. Intraluminal involvement of the larynx and trachea by thyroid cancer. Am J Surg 1974; 128: 500-4
- Cody 3rd HS, Shah JP. Locally invasive, well-differentiated thyroid cancer. 22 years' experience at memorial sloan-kettering cancer center. Am J Surg 1981; 142: 480-3
- Falk SA, McCaffrey TV. Management of the recurrent laryngeal nerve in suspected and proven thyroid cancer. Otolaryngol Head Neck Surg 1995; 113: 42-8
- Weber AL, Randolph G, Aksoy FG. The thyroid and parathyroid glands. CT and MR imaging and correlation with pathology and clinical findings. Radiol Clin North Am 2000; 38: 1105-29
- Seo YL, Yoon DY, Lim KJ, Cha JH, Yun EJ, Choi CS. et al. Locally advanced thyroid cancer: Can CT help in prediction of extrathyroidal invasion to adjacent structures?. Am J Roentgenol 2010; 195: W240-4
- Shin DH, Mark EJ, Suen HC, Grillo HC. Pathologic staging of papillary carcinoma of the thyroid with airway invasion based on the anatomic manner of extension to the trachea: A clinicopathologic study based on 22 patients who underwent thyroidectomy and airway resection. Hum Pathol 1993; 24: 866-70
- Takashima S, Takayama F, Wang J, Kobayashi S, Kadoya M. Using MR imaging to predict invasion of the recurrent laryngeal nerve by thyroid carcinoma. AJR Am J Roentgenol 2003; 180: 837-42
- Roychowdhury S, Loevner LA, Yousem DM, Chalian A, Montone KT. MR imaging for predicting neoplastic invasion of the cervical esophagus. AJNR Am J Neuroradiol 2000; 21: 1681-7
- Ohshima A, Yamashita H, Noguchi S, Uchino S, Watanabe S, Toda M. et al. Usefulness of endoscopic ultrasonography (EUS) in diagnosing esophageal infiltration of thyroid cancer. J Endocrinol Invest 2001; 24: 564-9
- Koike E, Yamashita H, Noguchi S, Ohshima A, Yamashita H, Watanabe S. et al. Endoscopic ultrasonography in patients with thyroid cancer: Its usefulness and limitations for evaluating esophagopharyngeal invasion. Endoscopy 2002; 34: 457-60
- Wang LY, Nixon IJ, Patel SG, Palmer FL, Tuttle RM, Shaha A. et al. Operative management of locally advanced, differentiated thyroid cancer. Surgery 2016; 160: 738-46
- Bai B, Chen W. Protective effects of intraoperative nerve monitoring (IONM) for recurrent laryngeal nerve injury in thyroidectomy: Meta-analysis. Sci Rep 2018; 8: 7761
- Tae K. Cost-effectiveness of intraoperative neural monitoring in thyroid surgery: Comment on “analyzing cost-effectiveness of neural-monitoring in recurrent laryngeal nerve recovery course in thyroid surgery”. Gland Surg 2019; 8: 304-6
- Wang T, Kim HY, Wu CW, Rausei S, Sun H, Pergolizzi FP. et al. Analyzing cost-effectiveness of neural-monitoring in recurrent laryngeal nerve recovery course in thyroid surgery. Int J Surg 2017; 48: 180-8
- Schneider R, Machens A, Randolph GW, Kamani D, Lorenz K, Dralle H. Opportunities and challenges of intermittent and continuous intraoperative neural monitoring in thyroid surgery. Gland Surg 2017; 6: 537-45
- Nishida T, Nakao K, Hamaji M, Kamiike W, Kurozumi K, Matsuda H. Preservation of recurrent laryngeal nerve invaded by differentiated thyroid cancer. Ann Surg 1997; 226: 85-91
- Chiang FY, Wang LF, Huang YF, Lee KW, Kuo WR. Recurrent laryngeal nerve palsy after thyroidectomy with routine identification of the recurrent laryngeal nerve. Surgery 2005; 137: 342-7
- Wu CW, Dionigi G, Barczynski M, Chiang FY, Dralle H, Schneider R. International neuromonitoring study group guidelines 2018: Part II: Optimal recurrent laryngeal nerve management for invasive thyroid cancer-incorporation of surgical, laryngeal, and neural electrophysiologic data. Laryngoscope 2018; 128: S18-27 et al. Suppl 3
- McCaffrey TV, Bergstralh EJ, Hay ID. Locally invasive papillary thyroid carcinoma: 1940-1990. Head Neck 1994; 16: 165-72
- McCarty TM, Kuhn JA, Williams Jr. WL, Ellenhorn JD, O'Brien JC, Preskitt JT. et al. Surgical management of thyroid cancer invading the airway. Ann Surg Oncol 1997; 4: 403-8
- Nishida T, Nakao K, Hamaji M. Differentiated thyroid carcinoma with airway invasion: Indication for tracheal resection based on the extent of cancer invasion. J Thorac Cardiovasc Surg 1997; 114: 84-92
- Friedman M, Danielzadeh JA, Caldarelli DD. Treatment of patients with carcinoma of the thyroid invading the airway. Arch Otolaryngol Head Neck Surg 1994; 120: 1377-81
- Farahati J, Reiners C, Stuschke M, Müller SP, Stüben G, Sauerwein W. et al. Differentiated thyroid cancer. Impact of adjuvant external radiotherapy in patients with perithyroidal tumor infiltration (stage pT4). Cancer 1996; 77: 172-80
- Fussey JM, Crunkhorn R, Tedla M, Weickert MO, Mehanna H. External beam radiotherapy in differentiated thyroid carcinoma: A systematic review. Head Neck 2016; 38 Suppl 1 E2297-305
- Terezakis SA, Lee KS, Ghossein RA, Rivera M, Tuttle RM, Wolden SL. et al. Role of external beam radiotherapy in patients with advanced or recurrent nonanaplastic thyroid cancer: Memorial sloan-kettering cancer center experience. Int J Radiat Oncol Biol Phys 2009; 73: 795-801
- Tsang RW, Brierley JD, Simpson WJ, Panzarella T, Gospodarowicz MK, Sutcliffe SB. The effects of surgery, radioiodine, and external radiation therapy on the clinical outcome of patients with differentiated thyroid carcinoma. Cancer 1998; 82: 375-88
- Lin JD, Tsang NM, Huang MJ, Weng HF. Results of external beam radiotherapy in patients with well differentiated thyroid carcinoma. Jpn J Clin Oncol 1997; 27: 244-7
Address for correspondence
Publication History
Received: 09 April 2020
Accepted: 11 June 2020
Article
published
online:
17 May 2021
© 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor,
Sector
2, Noida-201301 UP, India

| Figure 1:Contrast enhanced computed tomography scan of locally advanced thyroid cancer with disease infiltrating into the trachea without mucosal breach (Shin III). (Orange – tumour outline, Blue – Tracheal cartilage and Pink – Tracheal mucosa)

| Figure.2:Shin staging: Thyroid cancers invading the trachea: (top left) Stage I, (bottom left) Stage II, (top right) Stage III, and (bottom right) Stage IV (diagram conceptualized from Shin et al. [7])
References
- Britto E, Shah S, Parikh DM, Rao RS. Laryngotracheal invasion by well-differentiated thyroid cancer: Diagnosis and management. J Surg Oncol 1990; 44: 25-31
- Djalilian M, Beahrs OH, Devine KD, Weiland LH, DeSanto LW. Intraluminal involvement of the larynx and trachea by thyroid cancer. Am J Surg 1974; 128: 500-4
- Cody 3rd HS, Shah JP. Locally invasive, well-differentiated thyroid cancer. 22 years' experience at memorial sloan-kettering cancer center. Am J Surg 1981; 142: 480-3
- Falk SA, McCaffrey TV. Management of the recurrent laryngeal nerve in suspected and proven thyroid cancer. Otolaryngol Head Neck Surg 1995; 113: 42-8
- Weber AL, Randolph G, Aksoy FG. The thyroid and parathyroid glands. CT and MR imaging and correlation with pathology and clinical findings. Radiol Clin North Am 2000; 38: 1105-29
- Seo YL, Yoon DY, Lim KJ, Cha JH, Yun EJ, Choi CS. et al. Locally advanced thyroid cancer: Can CT help in prediction of extrathyroidal invasion to adjacent structures?. Am J Roentgenol 2010; 195: W240-4
- Shin DH, Mark EJ, Suen HC, Grillo HC. Pathologic staging of papillary carcinoma of the thyroid with airway invasion based on the anatomic manner of extension to the trachea: A clinicopathologic study based on 22 patients who underwent thyroidectomy and airway resection. Hum Pathol 1993; 24: 866-70
- Takashima S, Takayama F, Wang J, Kobayashi S, Kadoya M. Using MR imaging to predict invasion of the recurrent laryngeal nerve by thyroid carcinoma. AJR Am J Roentgenol 2003; 180: 837-42
- Roychowdhury S, Loevner LA, Yousem DM, Chalian A, Montone KT. MR imaging for predicting neoplastic invasion of the cervical esophagus. AJNR Am J Neuroradiol 2000; 21: 1681-7
- Ohshima A, Yamashita H, Noguchi S, Uchino S, Watanabe S, Toda M. et al. Usefulness of endoscopic ultrasonography (EUS) in diagnosing esophageal infiltration of thyroid cancer. J Endocrinol Invest 2001; 24: 564-9
- Koike E, Yamashita H, Noguchi S, Ohshima A, Yamashita H, Watanabe S. et al. Endoscopic ultrasonography in patients with thyroid cancer: Its usefulness and limitations for evaluating esophagopharyngeal invasion. Endoscopy 2002; 34: 457-60
- Wang LY, Nixon IJ, Patel SG, Palmer FL, Tuttle RM, Shaha A. et al. Operative management of locally advanced, differentiated thyroid cancer. Surgery 2016; 160: 738-46
- Bai B, Chen W. Protective effects of intraoperative nerve monitoring (IONM) for recurrent laryngeal nerve injury in thyroidectomy: Meta-analysis. Sci Rep 2018; 8: 7761
- Tae K. Cost-effectiveness of intraoperative neural monitoring in thyroid surgery: Comment on “analyzing cost-effectiveness of neural-monitoring in recurrent laryngeal nerve recovery course in thyroid surgery”. Gland Surg 2019; 8: 304-6
- Wang T, Kim HY, Wu CW, Rausei S, Sun H, Pergolizzi FP. et al. Analyzing cost-effectiveness of neural-monitoring in recurrent laryngeal nerve recovery course in thyroid surgery. Int J Surg 2017; 48: 180-8
- Schneider R, Machens A, Randolph GW, Kamani D, Lorenz K, Dralle H. Opportunities and challenges of intermittent and continuous intraoperative neural monitoring in thyroid surgery. Gland Surg 2017; 6: 537-45
- Nishida T, Nakao K, Hamaji M, Kamiike W, Kurozumi K, Matsuda H. Preservation of recurrent laryngeal nerve invaded by differentiated thyroid cancer. Ann Surg 1997; 226: 85-91
- Chiang FY, Wang LF, Huang YF, Lee KW, Kuo WR. Recurrent laryngeal nerve palsy after thyroidectomy with routine identification of the recurrent laryngeal nerve. Surgery 2005; 137: 342-7
- Wu CW, Dionigi G, Barczynski M, Chiang FY, Dralle H, Schneider R. International neuromonitoring study group guidelines 2018: Part II: Optimal recurrent laryngeal nerve management for invasive thyroid cancer-incorporation of surgical, laryngeal, and neural electrophysiologic data. Laryngoscope 2018; 128: S18-27 et al. Suppl 3
- McCaffrey TV, Bergstralh EJ, Hay ID. Locally invasive papillary thyroid carcinoma: 1940-1990. Head Neck 1994; 16: 165-72
- McCarty TM, Kuhn JA, Williams Jr. WL, Ellenhorn JD, O'Brien JC, Preskitt JT. et al. Surgical management of thyroid cancer invading the airway. Ann Surg Oncol 1997; 4: 403-8
- Nishida T, Nakao K, Hamaji M. Differentiated thyroid carcinoma with airway invasion: Indication for tracheal resection based on the extent of cancer invasion. J Thorac Cardiovasc Surg 1997; 114: 84-92
- Friedman M, Danielzadeh JA, Caldarelli DD. Treatment of patients with carcinoma of the thyroid invading the airway. Arch Otolaryngol Head Neck Surg 1994; 120: 1377-81
- Farahati J, Reiners C, Stuschke M, Müller SP, Stüben G, Sauerwein W. et al. Differentiated thyroid cancer. Impact of adjuvant external radiotherapy in patients with perithyroidal tumor infiltration (stage pT4). Cancer 1996; 77: 172-80
- Fussey JM, Crunkhorn R, Tedla M, Weickert MO, Mehanna H. External beam radiotherapy in differentiated thyroid carcinoma: A systematic review. Head Neck 2016; 38 Suppl 1 E2297-305
- Terezakis SA, Lee KS, Ghossein RA, Rivera M, Tuttle RM, Wolden SL. et al. Role of external beam radiotherapy in patients with advanced or recurrent nonanaplastic thyroid cancer: Memorial sloan-kettering cancer center experience. Int J Radiat Oncol Biol Phys 2009; 73: 795-801
- Tsang RW, Brierley JD, Simpson WJ, Panzarella T, Gospodarowicz MK, Sutcliffe SB. The effects of surgery, radioiodine, and external radiation therapy on the clinical outcome of patients with differentiated thyroid carcinoma. Cancer 1998; 82: 375-88
- Lin JD, Tsang NM, Huang MJ, Weng HF. Results of external beam radiotherapy in patients with well differentiated thyroid carcinoma. Jpn J Clin Oncol 1997; 27: 244-7


 PDF
PDF  Views
Views  Share
Share

