Analytical Study on the Efficacy of Neoadjuvant Chemotherapy Using a Combination of Methotrexate, Bleomycin, and Cisplatin in the Management of Advanced Squamous Cell Carcinoma of the Buccal Mucosa
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(03): 345-348
DOI: DOI: 10.4103/ijmpo.ijmpo_123_17
Abstract
Context: Cancers of the buccal mucosa (CaBM) predominate in India with late- stage diagnosis and poor survival, necessitating optimal management. Aim: Our study aimed at testing the efficacy of combination neoadjuvant chemotherapy (NACT) using cisplatin (CIS), bleomycin (BL) and methotrexate (MTX) for reducing tumour volume prior to surgery. Methodology: Patients with advanced CaBM (stage III, IV, n = 100) were administered 6 rounds of NACT with CIS, BL and MTX. Responses, toxicity and 6-month follow-up was monitored statistically to determine persistence of response. Results: A significant number of patients showed objective response as either complete or partial tumour regression with subjective response as reduced trismus, pain, salivation and foul odour. Moreover, there was mild associated toxicity and tumour regression continued in most patients even after 6-month follow-up. Conclusion: Our study indicates that NACT with CIS, BL and MTX offers a good therapeutic alternative in terms of significant objective and subjective responses, low toxicity, affordable costs and persistent responses.
Publication History
Article published online:
04 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Context:
Cancers of the buccal mucosa (CaBM) predominate in India with late- stage diagnosis and poor survival, necessitating optimal management. Aim: Our study aimed at testing the efficacy of combination neoadjuvant chemotherapy (NACT) using cisplatin (CIS), bleomycin (BL) and methotrexate (MTX) for reducing tumour volume prior to surgery.
Methodology:
Patients with advanced CaBM (stage III, IV, n = 100) were administered 6 rounds of NACT with CIS, BL and MTX. Responses, toxicity and 6-month follow-up was monitored statistically to determine persistence of response.
Results:
A significant number of patients showed objective response as either complete or partial tumour regression with subjective response as reduced trismus, pain, salivation and foul odour. Moreover, there was mild associated toxicity and tumour regression continued in most patients even after 6-month follow-up.
Conclusion:
Our study indicates that NACT with CIS, BL and MTX offers a good therapeutic alternative in terms of significant objective and subjective responses, low toxicity, affordable costs and persistent responses.
Introduction
Buccal mucosal cancers (CaBM) are prevalent in India, primarily associated with etiological risk factors such as tobacco, alcohol, betel quid chewing, and infection with human papilloma virus and having poor survival.[1]
Although surgery/radiation/chemotherapy are effective methods of treatment, most CaBM are diagnosed late, where neoadjuvant chemotherapy followed by surgery is often the choice of treatment of locally advanced, unresectable disease, to reduce the tumor size for successful resection and improved outcomes.[2]
Several studies have demonstrated the efficacy of cisplatin, either alone, or in combination with methotrexate, 5-fluorouracil, vincristine, bleomycin, immunotherapy, etc., in CaBM.[3] Cisplatin combined with methotrexate and bleomycin have been used in advanced carcinomas of the male genital tract.[4] However, to the best of our knowledge, there are no studies using a combination of cisplatin, bleomycin, and methotrexate in neoadjuvant settings for treatment of advanced CaBM.
Our study focuses on Indian patients presenting advanced, inoperable stages CaBM. Neoadjuvant chemotherapy using a combination of cisplatin, bleomycin, and methotrexate was administered at a fixed dose with follow up for 6 months to assess its overall effectiveness. Our results indicate that combination chemotherapy yielded excellent and persistent objective response, in addition to cost effectiveness and tolerance.
Materials and Methods
Study population
The study included 100 patients who had visited the out-patient departments at Chittaranjan National Cancer Institute, Kolkata and M. G. M. Medical College and Lions Seva Kendra Hospital, Kishan gunj, Bihar, from September 2009 to August 2011, for biopsy and/or surgery. Eligibility criteria were pathologically confirmed, untreated patients harboring advanced stages of CaBM (Stages III and IV). Exclusion criteria included patients with lower stage, tuberculosis, renal insufficiency, and pregnancy to negate the effect of these factors on treatment outcomes. Similarly, patients with severe anemia, hookworm, and enlarged lymph nodes were given necessary treatment before inclusion in the study.
Relevant clinical history including lifetime exposure to tobacco and other habits were obtained from patients through an oral questionnaire with written informed consent, as well as clearance from hospital authorities and institutional ethical committees before the study.
Examinations, investigations, and confirmation of stage and grade of the tumor
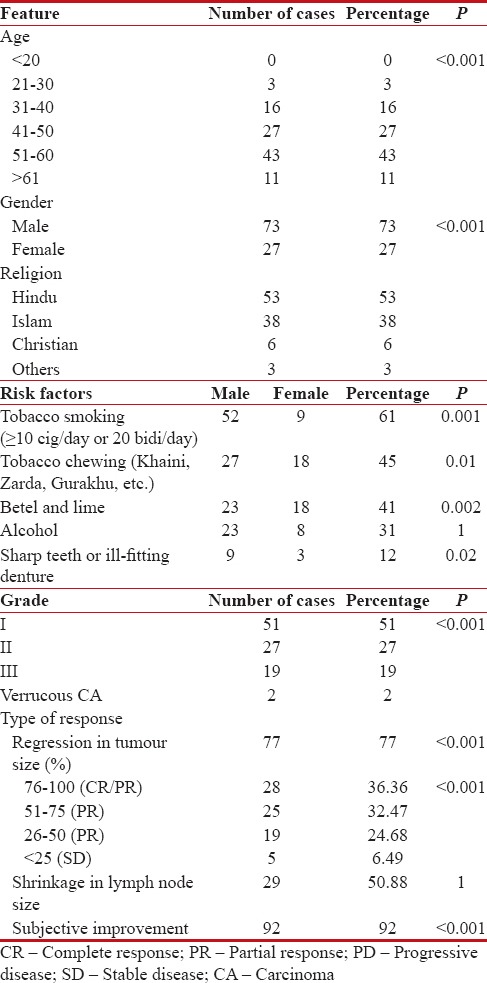
Patients were subjected to an initial local examination using meticulous calliper measurements to assess tumor volume, followed by routine examinations, such as X-rays, blood, urine, and stool, before the commencement of treatment, along with biopsy and confirmation of status of lymph nodes using fine-needle aspiration cytology. All tumors were classified in TNM stages based on the American Joint Committee on Cancer manual and histopathologically graded.[1] Patient details are presented in Table 1.
Table 1
Demography of the patients and therapeutic responses
|
Chemotherapy regimen
A combination of intravenous methotrexate, bleomycin, and cisplatin was used in the study, in the doses 60 mg, 15 units and 70 mg/m2 of body surface, respectively. Body surface area was calculated using the DuBois formula,[5] with average 1.73 m2, 1.7 m2, and 1.6 m2 for adults, men and women, respectively. Chemotherapy was given in 6 cycles, each cycle comprising 21 days [Figure 1a]. Supportive aid in the form of vitamins, antibiotics, bronchodilators, steroids, calcium leucovorin, blood transfusion, and electrolyte supplements were given to the patients during treatment to counter adverse side effects, along with maintenance and monitoring of a constant level of blood parameters (hemoglobin, 10 g %; WBC, 4000/mm3; platelet, >100,000/mm3; urea, <40>
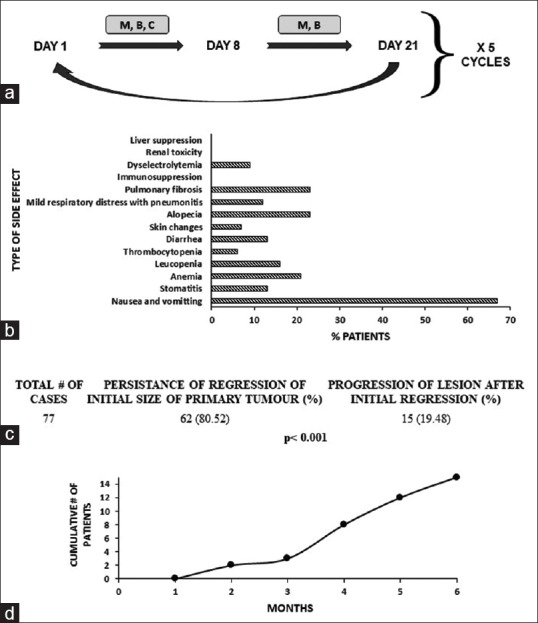
| Figure 1:Chemotherapy regimen, and follow up of responders after 6 months. (a) Chemotherapy was given to patients on days 1, 8, and 21, and the dose repeated for 5 cycles. M: Methotrexate; B: Bleomycin; C: Cisplatin. (b) Toxicities associated with chemotherapy were mild and mostly nausea and vomiting. (c) Status of the lesion in responders after a follow up of 6 months. P value (Fisher's exact) represents level of significance. (d) Time point (in months) of progression of the lesion after initial response to chemotherapy in 15 patients
Evaluation of treatment
Patients were routinely assessed weekly for treatment response, treatment-induced side effects, inspection and palpation of the primary tumor and associated lymph nodes, complete blood count, and body weight. Liver and renal functions were evaluated monthly.
Objective responses were assessed according to the criteria of the World Health Organization (WHO).[6] Complete response (CR) is defined as disappearance of all target lesions, partial response (PR) as at least 30% reduction in the sum of diameters of target lesion, progressive disease (PD) as at least 20% increase in the sum of diameters of target lesion and stable disease (SD) neither sufficient increase for denomination of PD nor sufficient shrinkage to qualify for PR.[6] Toxicity was monitored according to the WHO criteria.[6]
Study end point
The end point selected was 6 months, or the time from randomization to death. Data were censored either if the patient was lost in follow up or was alive till the end point.
Statistical analysis
Estimation of associated risk was determined by Odd's ratio using Fisher's exact test. Chi-square for trend was used to detect the linear variation with grades and stages of tumours. All tests were two-tailed, with a confidence interval of 95% and probability P < 0 href="https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5686980/#ref1" rid="ref1" class=" bibr popnode tag_hotlink tag_tooltip" id="__tag_649899512" role="button" aria-expanded="false" aria-haspopup="true" xss=removed>1]
Results
Patients presented with an older age and with associated risk factors
There as a significant association between CaBM and patient age with predominantly male patients. Moreover, most patients presented with either one or more risk factors, with trend toward lower grades of the disease [Table 1].
Combination chemotherapy yielded good response after initial completion, although with minor associated toxicity
A significant number of patients showed regression of the tumor after immediate completion of chemotherapy with most patients presenting with 51%–100% reduction in tumor size [Table 1]. Most patients showed either CR or PR. Moreover, a significant number of patients showed subjective improvement in terms of alleviation of pain and salivation, decrease in trismus, and decrease of foul odor. However, only about half of the patients bearing enlarged lymph nodes draining from the tumor site showed shrinkage of the same [Table 1].
Therapy-associated toxicity was mostly mild and well tolerated. The most common side effect included nausea and vomiting (67%), flowed by alopecia (23%), pulmonary fibrosis (23%), and anemia (21%). Patients recovered from toxicity effects soon after transient withdrawal of chemotherapy drugs or administration of supportive care [Figure 1b].
Combination chemotherapy provided significantly better objective response after 6-month follow up
Most patients showed persistence of regression of the initial tumor even after a follow up of 6 months [Figure 1c]. However, a small number of patients demonstrated progression of the lesion after initial regression over time [Figure 1c and andd].d]. There were no deaths due to disease, and a single case was lost in follow up (data not shown).
Discussion
The importance of combination chemotherapy in the treatment of advanced cancers is well documented. Previous research has shown the effectiveness of methotrexate, bleomycin, and cisplatin separately for palliation and prolongation of survival, along with alleviation of symptoms in patients presenting with advanced squamous cell carcinomas of the oral cavity.[3] The present study thus aimed to understand how the combination of the three drugs would benefit patients with advanced CaBM, both immediately and after a 6-month follow up.
The study indicates maximum disease burden in the age group of 51–60 years (43%) with significantly higher number of affected males and with lower grade. Similar results of CaBM have been reported in different parts of India.[7]
Most patients were habitual users of tobacco (smoking, chewing), betel quid, and lime or alcohol. Such high-risk habits are highly prevalent in India and throughout the Indian subcontinent and South-East Asia.[8]
The combination chemotherapy using methotrexate, bleomycin, and cisplatin gave a significantly high rate of tumor regression, with a significant number of patients showing either CR or PR. These results are comparable by to those done by other researchers separately using these drugs.[3,4] However, to the best of our knowledge, this is the first study for the eastern part of India where this combination of drugs was used in neoadjuvant settings to induce shrinkage of advanced CaBM before management and/or radiation.
Shrinkage of enlarged lymph nodes, as observed by us, corroborate with other studies.[9] Similarly, subjective improvement in terms of alleviation of pain, reduction of salivation and drooling, decrease in foul odor and reduction of trismus was observed in our studies, similar to others.[10] Most patients showed regression of the tumor after a follow up of 6 months, thus indicating the effectiveness of the combination therapy.
Conclusion
Our results using neoadjuvant chemotherapy using methotrexate, bleomycin, and cisplatin in the treatment of advanced carcinomas of the buccal mucosa thus indicate the effectiveness of the drug combination, not only in terms of tumour regression and enhanced subjective responses, even after 6 months of completion of the chemotherapy regimen but also in being cost effective and producing a low level of associated toxicity. This drug combination can thus be used for the treatment of inoperable cases of oral cancers with superior prognostic implications. However, further studies using a larger cohort and with longer follow-up is warranted.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
Acknowledgments
The authors would like to thank the Directors, Chittaranjan National Cancer Institute, Kolkata and M. G. M. Medical College and Lions Seva Kendra Hospital, Kishan gunj, Bihar for their support and to all participants of the study for their cooperation.
References
- Sarkar S, Alam N, Chakraborty J, Biswas J, Mandal SS, Roychoudhury S, et al. Human papilloma virus (HPV) infection leads to the development of head and neck lesions but offers better prognosis in malignant Indian patients. Med Microbiol Immunol 2017;206:267-76.
- Sarkar S, Maiti GP, Jha J, Biswas J, Roy A, Roychoudhury S, et al. Reduction of proliferation and induction of apoptosis are associated with shrinkage of head and neck squamous cell carcinoma due to neoadjuvant chemotherapy. Asian Pac J Cancer Prev 2013;14:6419-25.
- Pendleton KP, Grandis JR. Cisplatin-based chemotherapy options for recurrent and/or metastatic squamous cell cancer of the head and neck. Clin Med Insights Ther 2013;2013.
- Hakenberg OW, Nippgen JB, Froehner M, Zastrow S, Wirth MP. Cisplatin, methotrexate and bleomycin for treating advanced penile carcinoma. BJU Int 2006;98:1225-7.
- Würthwein G, Krefeld B, Gerss J, Boos J. Carboplatin dosing in children: Calculation by different formulae. Onkologie 2011;34:16-22.
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47.
- Padma R, Paulraj S, Sundaresan S. Squamous cell carcinoma of buccal mucosa: Prevalence of clinicopathological pattern and its implications for treatment. SRM J Res Dent Sci 2017;8:9-13.
- Sharma M, Deshpandey A, Gupta N, Patel M. Retrospective analysis of oral cavity squamous cell carcinoma treated with surgery and adjuvent radiotherapy. Int J Res Med Sci 2016;4:1000-4.
- Kovács AF, Döbert N, Engels K. The effect of intraarterial high-dose cisplatin on lymph nodes in oral and oropharyngeal cancer. Indian J Cancer 2012;49:230-5.
- Turner L, Mupparapu M, Akintoye SO. Review of the complications associated with treatment of oropharyngeal cancer: A guide for the dental practitioner. Quintessence Int 2013;44:267-79.

| Figure 1:Chemotherapy regimen, and follow up of responders after 6 months. (a) Chemotherapy was given to patients on days 1, 8, and 21, and the dose repeated for 5 cycles. M: Methotrexate; B: Bleomycin; C: Cisplatin. (b) Toxicities associated with chemotherapy were mild and mostly nausea and vomiting. (c) Status of the lesion in responders after a follow up of 6 months. P value (Fisher's exact) represents level of significance. (d) Time point (in months) of progression of the lesion after initial response to chemotherapy in 15 patients
References
- Sarkar S, Alam N, Chakraborty J, Biswas J, Mandal SS, Roychoudhury S, et al. Human papilloma virus (HPV) infection leads to the development of head and neck lesions but offers better prognosis in malignant Indian patients. Med Microbiol Immunol 2017;206:267-76.
- Sarkar S, Maiti GP, Jha J, Biswas J, Roy A, Roychoudhury S, et al. Reduction of proliferation and induction of apoptosis are associated with shrinkage of head and neck squamous cell carcinoma due to neoadjuvant chemotherapy. Asian Pac J Cancer Prev 2013;14:6419-25.
- Pendleton KP, Grandis JR. Cisplatin-based chemotherapy options for recurrent and/or metastatic squamous cell cancer of the head and neck. Clin Med Insights Ther 2013;2013.
- Hakenberg OW, Nippgen JB, Froehner M, Zastrow S, Wirth MP. Cisplatin, methotrexate and bleomycin for treating advanced penile carcinoma. BJU Int 2006;98:1225-7.
- Würthwein G, Krefeld B, Gerss J, Boos J. Carboplatin dosing in children: Calculation by different formulae. Onkologie 2011;34:16-22.
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47.
- Padma R, Paulraj S, Sundaresan S. Squamous cell carcinoma of buccal mucosa: Prevalence of clinicopathological pattern and its implications for treatment. SRM J Res Dent Sci 2017;8:9-13.
- Sharma M, Deshpandey A, Gupta N, Patel M. Retrospective analysis of oral cavity squamous cell carcinoma treated with surgery and adjuvent radiotherapy. Int J Res Med Sci 2016;4:1000-4.
- Kovács AF, Döbert N, Engels K. The effect of intraarterial high-dose cisplatin on lymph nodes in oral and oropharyngeal cancer. Indian J Cancer 2012;49:230-5.
- Turner L, Mupparapu M, Akintoye SO. Review of the complications associated with treatment of oropharyngeal cancer: A guide for the dental practitioner. Quintessence Int 2013;44:267-79.


 PDF
PDF  Views
Views  Share
Share

