Anaplastic Multiple Myeloma- a Morphological Challenge – A Case Series
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2020; 41(03): 430-433
DOI: DOI: 10.4103/ijmpo.ijmpo_96_19
Abstract
Anaplastic multiple myeloma (AMM) is a rare and aggressive variant of plasma cell neoplasm. It is relatively treatment resistant when compared to conventional myeloma. It can arise de novo or transform from a preexisting plasma cell myeloma. Morphologically, AMM is recognized by the presence of immature plasma cells and pleomorphic, multilobated cells. Here, we present three cases of AMM.
Keywords
Anaplastic myeloma - anaplastic variant of multiple myeloma - bizarre plasma cells - multilobated plasma cellsPublication History
Received: 13 April 2019
Accepted: 03 January 2020
Article published online:
28 June 2021
© 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Anaplastic multiple myeloma (AMM) is a rare and aggressive variant of plasma cell neoplasm. It is relatively treatment resistant when compared to conventional myeloma. It can arise de novo or transform from a preexisting plasma cell myeloma. Morphologically, AMM is recognized by the presence of immature plasma cells and pleomorphic, multilobated cells. Here, we present three cases of AMM.
Keywords
Anaplastic myeloma - anaplastic variant of multiple myeloma - bizarre plasma cells - multilobated plasma cellsIntroduction
Anaplastic multiple myeloma (AMM) is a very rare morphological variant of multiple myeloma. It has an aggressive clinical course and poor prognosis. It is relatively more treatment resistant when compared to conventional myeloma. It can arise de novo or transform from a preexisting plasma cell myeloma.[1],[2]
Morphologically, AMM is recognized by the presence of immature plasma cells and pleomorphic, multilobated cells. These cells can be mistaken for carcinoma or lymphoma cells or even at times megakaryocytes. Here, we present three cases of AMM.[3]
Case Reports
Case 1
An 85-year-old male who was diagnosed to have chronic kidney disease presented with the symptoms of anemia and back pain. Laboratory investigations showed anemia (hemoglobin [Hb]: 6.6 g/dl), erythrocyte sedimentation rate (ESR) of 120 mm, and elevated renal parameters (blood urea – 87 mg/dl and serum creatinine – 4.38 mg/dl). Serum albumin was 3.1 g/dl and globulin was 4.8 g/dl. Peripheral smear showed normocytic anemia with exaggerated rouleaux formation [Figure 1].
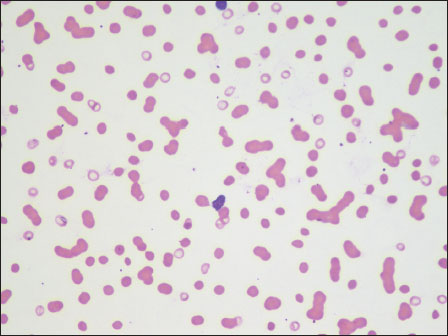
| Figure 1:Case 1: Peripheral smear with exaggerated rouleaux formation (×40)
Bone marrow aspiration showed hypercellular marrow with significant plasmacytosis (plasma cells −72%). Also seen were many large cells with eccentrically placed hyperchromatic nuclei exhibiting marked nuclear lobulations and indentations. Subsequently, serum protein electrophoresis was done which demonstrated a discrete M band.
Case 2
A 61-year-old male, a diagnosed case of chronic kidney disease, presented with severe fatigue and bony tenderness. Blood investigations showed elevated renal parameters (blood urea – 95 mg/dl and serum creatinine – 7.42 mg/dl). There were significant albumin–globulin ratio reversal (serum albumin – 2.1 g/dl and globulin – 12.3 g/dl) and markedly elevated ESR (109 mm/h). Peripheral smear showed normocytic anemia (Hb – 4.4 g/dl), thrombocytopenia (87,000/mm3), white blood cell count of 4400/mm3, increased rouleaux formation, and presence of 4%-atypical cells [Figure 2].
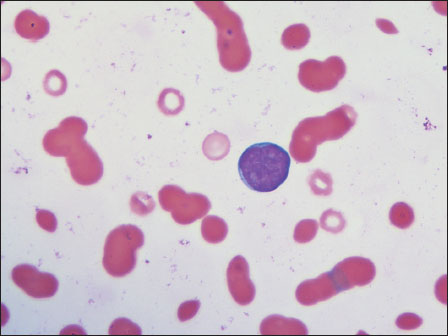
| Figure.2:Case 2: Peripheral smear with a blast cell (×100)
Serum protein electrophoresis showed a significant fall in albumin level and a steep increase in the gamma globulin region suggestive of “M band.” Bone marrow aspiration showed hypercellular marrow particles with pleomorphic plasmacytoid cells with lobated nuclei along with a few mature, binucleate and multinucleate plasma cells. Hypercellular marrow spaces with sheets and singly scattered pleomorphic cells were observed on trephine biopsy. Plasmacytoid morphology was focally discernible [Figure 3]. The differentials included lymphoma versus myeloma. Immunohistochemical markers were done. The cells expressed CD138 and were negative for CD20 immunostain [Figure 4] and [Figure 5].{Figure 3}{Figure 4}{Figure 5}
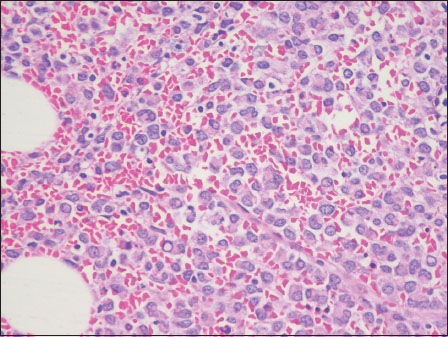
| Figure.3:Case 2: Bone marrow trephine showing sheets of plasma cells, anaplastic cells, and Dutcher bodies (H and E, ×40)
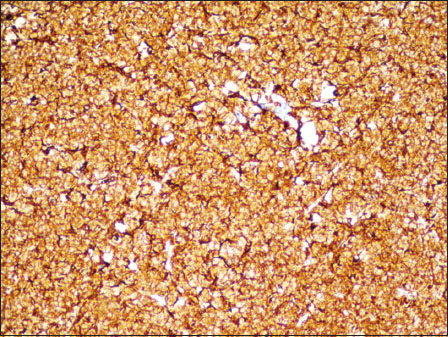
| Figure.4:Case 2: Bone marrow trephine with cells expressing membranous CD138 (immunohistochemistry × 40)

| Figure.5:Case 2: Bone marrow trephine showing the neoplastic cells negative for CD20 (immunohistochemistry ×40)
Case 3
A 70-year-old male presented with severe fatigue, loss of appetite, and weight loss. He was a known case of multiple myeloma diagnosed 6 years back. He was in remission following treatment. Peripheral smear showed pancytopenia with increased rouleaux formation and presence of plasma cells. Bone marrow studies were done to rule out recurrence. Bone marrow aspirate showed bizarre plasma cells [Figure 6] and [Figure 7]. The marrow spaces showed diffuse infiltration by plasmacytoid cells and bizarre cells with multilobated nuclei. The cells expressed membranous staining for CD138. The bone marrow features were consistent with the anaplastic transformation of a myeloma.

| Figure.6:Case 3: Bone marrow aspirate with bizarre plasma cells (×100)

| Figure.7:Case 3: Bone marrow aspirate with a plasma cell showing abnormal nuclear lobulation (×100)
Discussion
Multiple myeloma is a common hematological malignancy of the middle aged and elderly. AMM was originally used to describe high-grade transformation occurring as a terminal event in the malignant plasma cell clones having pleomorphic morphology and immature plasma cells.[3],[4] It has been referred by various names such as plasma cell sarcoma, aggressive-phase myeloma, and dysplastic myeloma. It is more commonly reported in younger adults, with the median age being 53 years, associated with predisposition to extramedullary sites and immunoglobulin A isotype.[2] Cases of anaplastic myeloma with poorly differentiated cells having extensive involvement of intra-abdominal and retroperitoneal sites have been reported in literature. AMM has morphological and flow cytometry features which are distinct from those of the conventional myeloma.[4]
Historically, this disease has been very resistant to chemotherapy. It has been suggested that this aggressive phase could result from a clonal evolution of the original malignant cell and not considered as the development of an independent new neoplasia.
All the three cases reported were seen in adults. None of them had any evidence of extramedullary disease. Two cases presented de novo, whereas the other was anaplastic transformation seen in a patient in remission after treatment.
Multiple myeloma exhibits a broad morphological variation ranging from mature plasmacytic to highly anaplastic variants. The anaplastic variant poses a morphological diagnostic challenge as it mimics carcinoma and lymphomas, especially when presenting de novo. The presence of large cells with nuclear budding may mimic a megakaryocyte in bone marrow samples.[5],[6]
Case 2 reported here had a very anaplastic morphology, and the diagnosis was made with the aid of immunohistochemical staining with CD138.
Diagnosing a case as anaplastic myeloma without ancillary techniques is difficult, especially when the histology shows primitive cells. One of our cases had an undifferentiated morphology.
Bone marrow findings varied among the three cases.
Case 1 showed 72%-plasma cells with the presence of few anaplastic cells. Whereas, case 2 showed predominantly large cells with pleomorphic morphology and scattered plasma cells.
Case 3 showed plasmacytoid cells with anaplastic morphology and occurred as a recurrence.
AMM, irrespective of presenting de novo or as progression, has been reported to have poor response to chemotherapy.[4]
Conclusion
Here, we present a series of three cases of anaplastic myeloma for the rarity of this entity and also for the fact that these cases were considerably different from the previously reported cases in having no evidence of extramedullary disease and presenting at an older age. Anaplastic myeloma needs to be considered in bone marrow aspirates with pleomorphic morphology in a patient with other supportive evidence of plasma cell dyscrasia.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Conflict of Interest
There are no conflicts of interest.
References
- Rodriguez C, Strowd RE, Grier DD. Anaplastic myeloma arising from myeloma: Successful management of two diseases in an individual. Blood 2017; 130: 5410
- Sethi S, Miller I. Plasma cell myeloma with anaplastic transformation. Blood 2016; 128: 2106
- Harankhedkar S, Gupta R, Rahman K. Pleomorphic multinucleated plasma cells simulating megakaryocytes in an anaplastic variant of myeloma. Turk J Haematol 2018; 35: 150-1
- Ammannagari N, Celotto K, Neppalli V, Lee K, Holstein SA. Anaplastic multiple myeloma: An aggressive variant with a poor response to novel therapies. Clin Lymphoma Myeloma Leuk 2016; 16: e129-31
- Rao S, Kar R, Pati HP. Anaplastic myeloma: A morphologic diagnostic dilemma. Indian J Hematol Blood Transfus 2008; 24: 188-9
- Fujimi A, Nagamachi Y, Yamauchi N, Kanisawa Y. Morphological transformation of myeloma cells into multilobated plasma cell nuclei within 7 days in a case of secondary plasma cell leukemia that finally transformed as anaplastic myeloma. Case Rep Hematol 2017; 2017: 5758368
Address for correspondence
Publication History
Received: 13 April 2019
Accepted: 03 January 2020
Article published online:
28
June 2021
© 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301
UP, India

| Figure 1:Case 1: Peripheral smear with exaggerated rouleaux formation (×40)

| Figure.2:Case 2: Peripheral smear with a blast cell (×100)

| Figure.3:Case 2: Bone marrow trephine showing sheets of plasma cells, anaplastic cells, and Dutcher bodies (H and E, ×40)

| Figure.4:Case 2: Bone marrow trephine with cells expressing membranous CD138 (immunohistochemistry × 40)

| Figure.5:Case 2: Bone marrow trephine showing the neoplastic cells negative for CD20 (immunohistochemistry ×40)

| Figure.6:Case 3: Bone marrow aspirate with bizarre plasma cells (×100)

| Figure.7:Case 3: Bone marrow aspirate with a plasma cell showing abnormal nuclear lobulation (×100)
References
- Rodriguez C, Strowd RE, Grier DD. Anaplastic myeloma arising from myeloma: Successful management of two diseases in an individual. Blood 2017; 130: 5410
- Sethi S, Miller I. Plasma cell myeloma with anaplastic transformation. Blood 2016; 128: 2106
- Harankhedkar S, Gupta R, Rahman K. Pleomorphic multinucleated plasma cells simulating megakaryocytes in an anaplastic variant of myeloma. Turk J Haematol 2018; 35: 150-1
- Ammannagari N, Celotto K, Neppalli V, Lee K, Holstein SA. Anaplastic multiple myeloma: An aggressive variant with a poor response to novel therapies. Clin Lymphoma Myeloma Leuk 2016; 16: e129-31
- Rao S, Kar R, Pati HP. Anaplastic myeloma: A morphologic diagnostic dilemma. Indian J Hematol Blood Transfus 2008; 24: 188-9
- Fujimi A, Nagamachi Y, Yamauchi N, Kanisawa Y. Morphological transformation of myeloma cells into multilobated plasma cell nuclei within 7 days in a case of secondary plasma cell leukemia that finally transformed as anaplastic myeloma. Case Rep Hematol 2017; 2017: 5758368


 PDF
PDF  Views
Views  Share
Share

